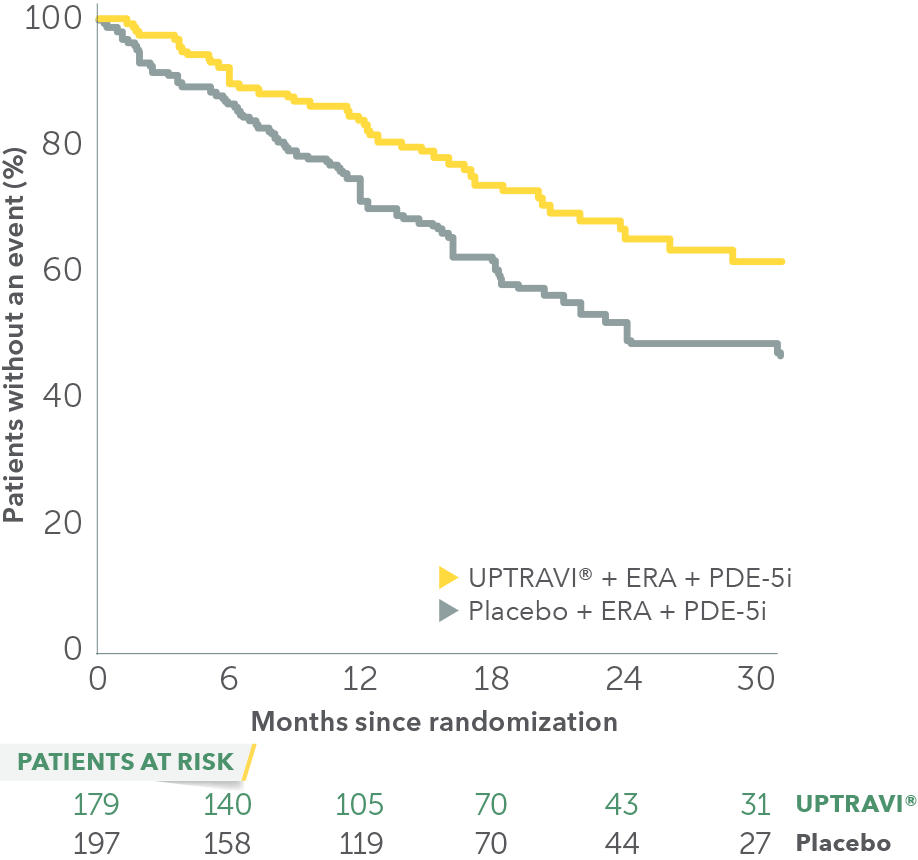
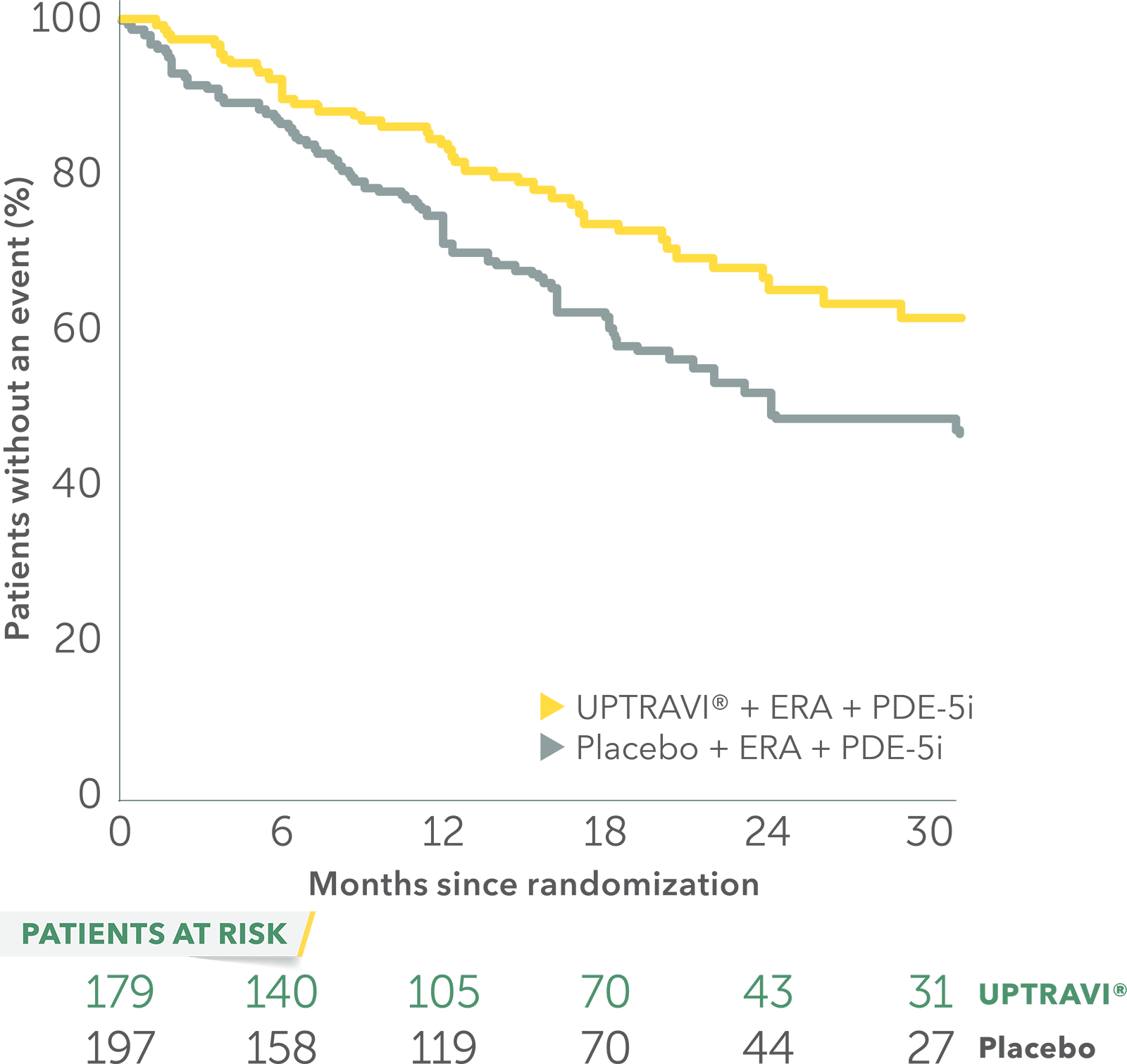
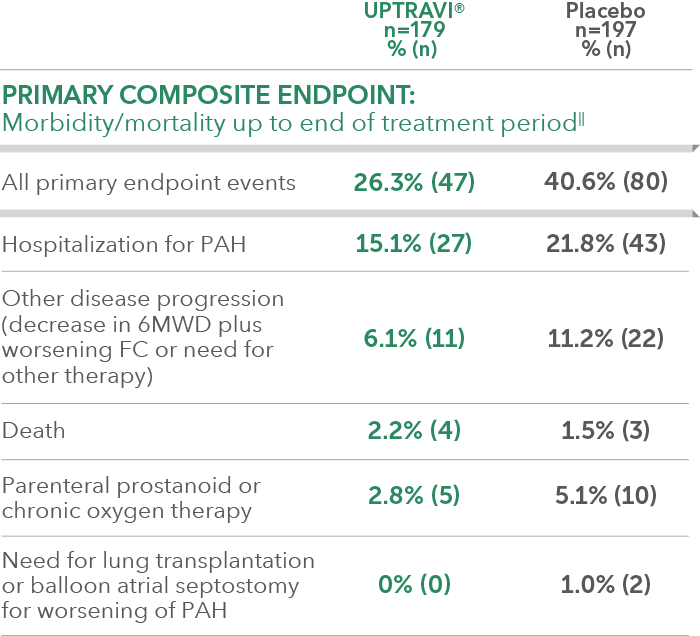
In an exploratory post hoc subgroup analysis, UPTRAVI® was associated with a: 37% RELATIVE RISK REDUCTION OF DISEASE PROGRESSION IN PATIENTS TREATED WITH UPTRAVI® + ERA + PDE-5i VS PLACEBO3*
Time to first disease progression event

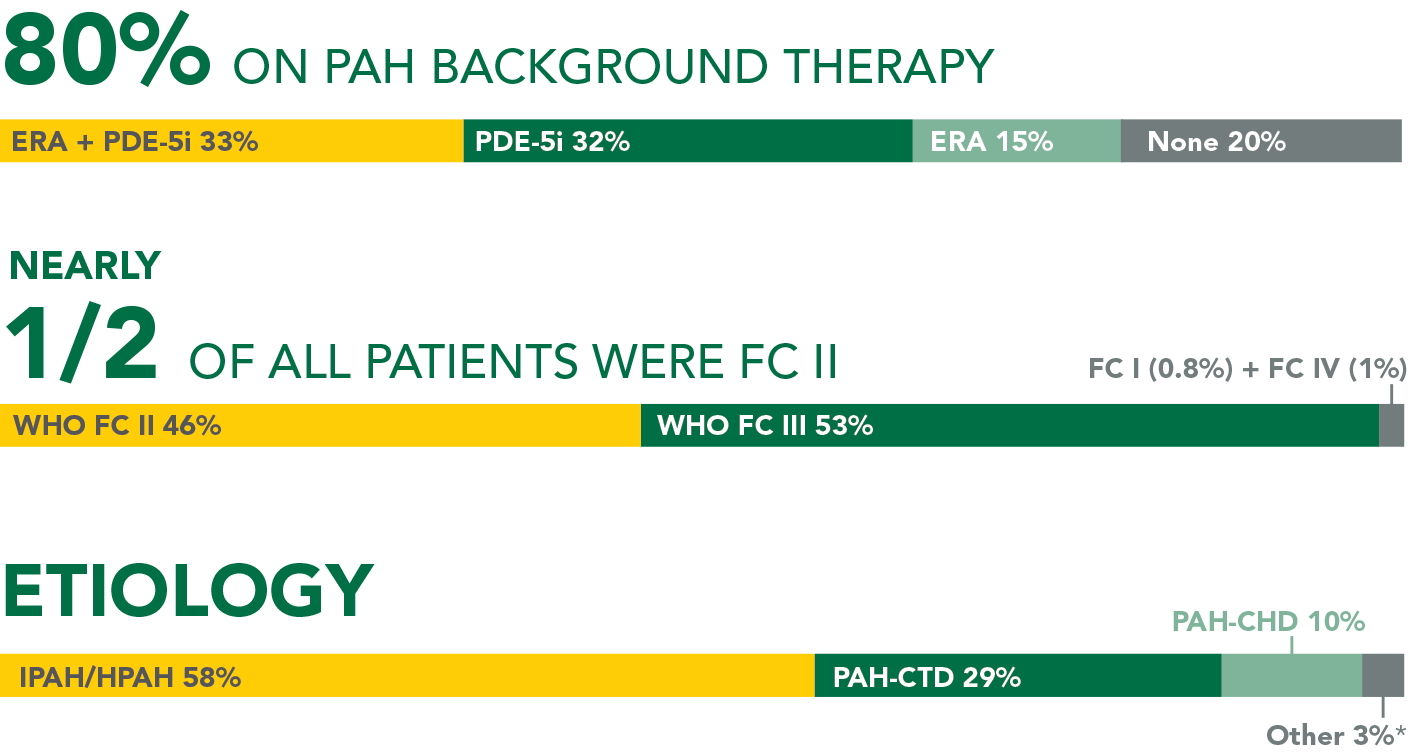
Baseline patient characteristics
- 33% (n=376) of all patients in GRIPHON were receiving an ERA and a PDE-5i at baseline (31% FC II and 68% FC III)†
- Etiology in triple-combination subgroup: IPAH/HPAH (64%), PAH-CTD (26%), PAH-CHD (5%), other (5%)‡
- Time from diagnosis: UPTRAVI® (4 years), placebo (3.6 years)
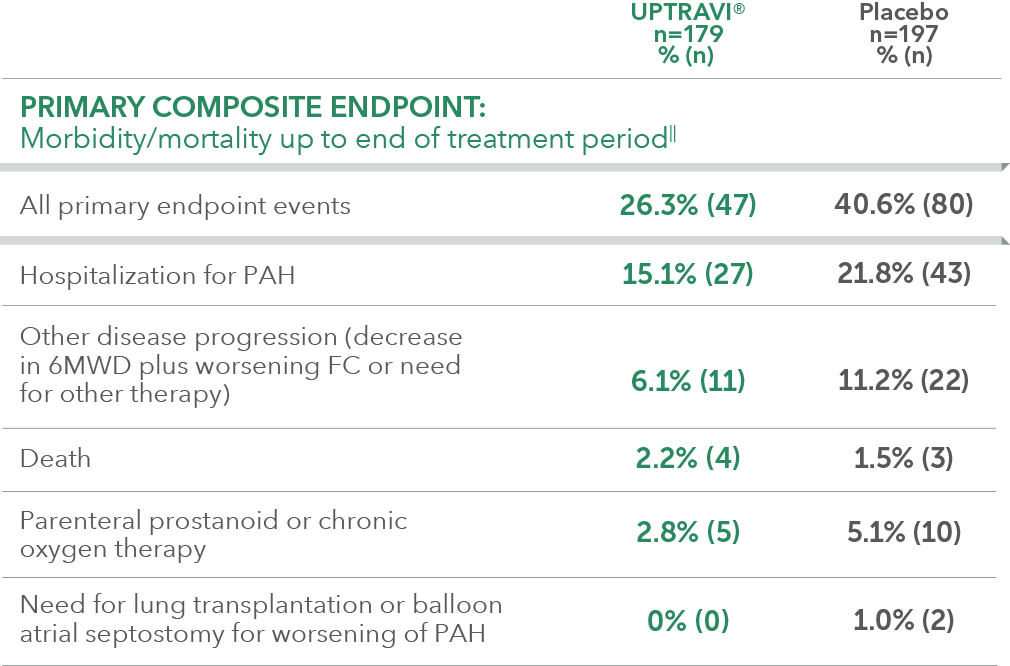
Summary of primary endpoint events in patients receiving ERA + PDE-5i at baseline
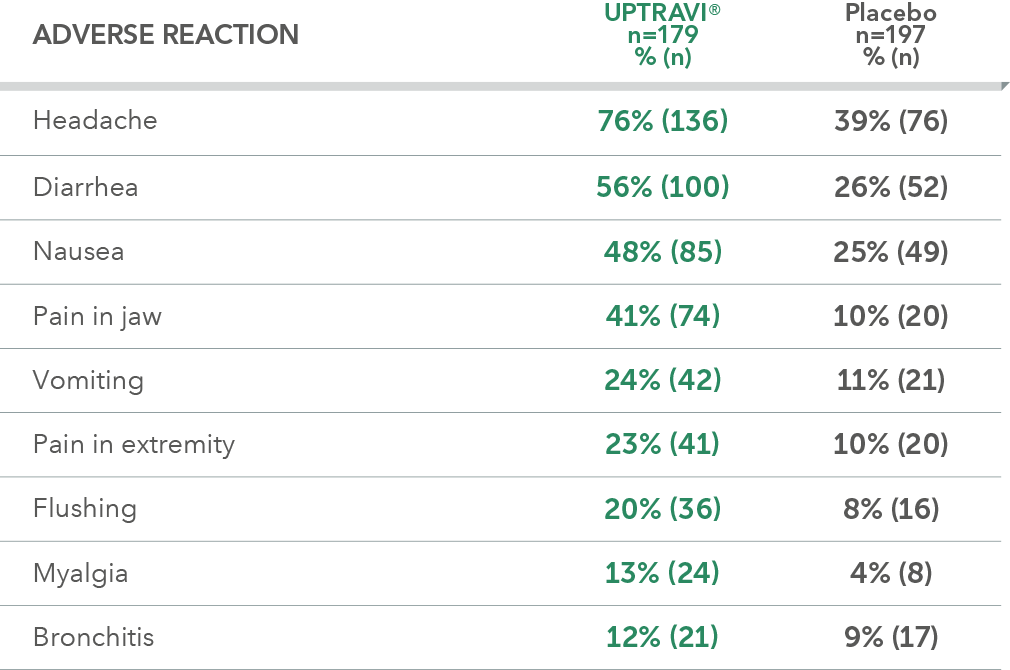
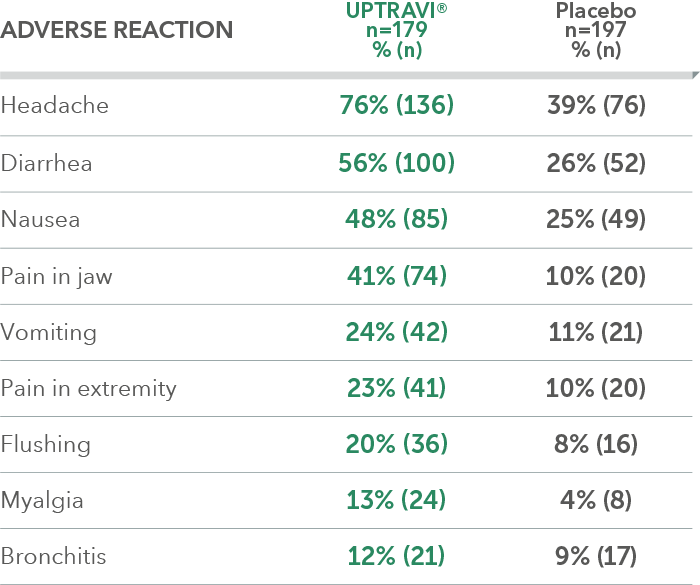
Adverse reactions in triple-combination subgroup occurring more frequently with UPTRAVI® compared with placebo by ≥3%
2022 ESC/ERS Guidelines: UPTRAVI® is the only prostacyclin pathway therapy to receive the highest-class recommendation (Class I, Level B)¶ for sequential triple- and dual-combination therapy4
Patients receiving an ERA and a PDE-5i at baseline were a prespecified subgroup for evaluation of the GRIPHON primary endpoint; however, the more detailed analyses described on this page are exploratory and post hoc. Sample size should be considered and results should be interpreted with caution.
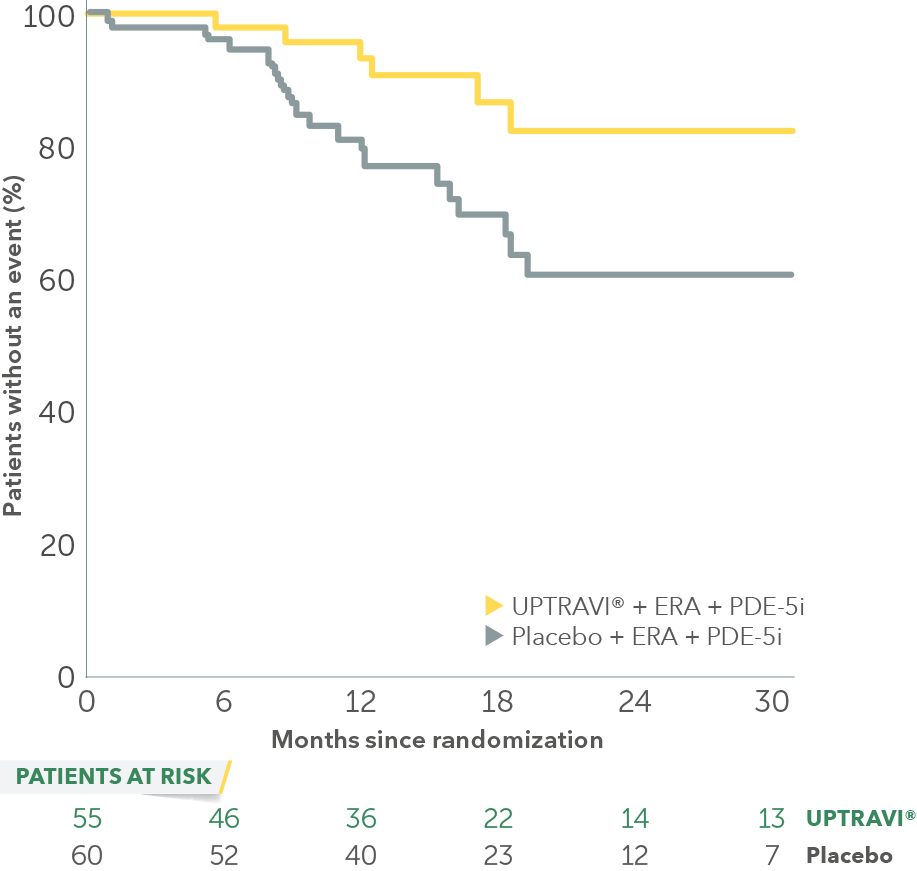
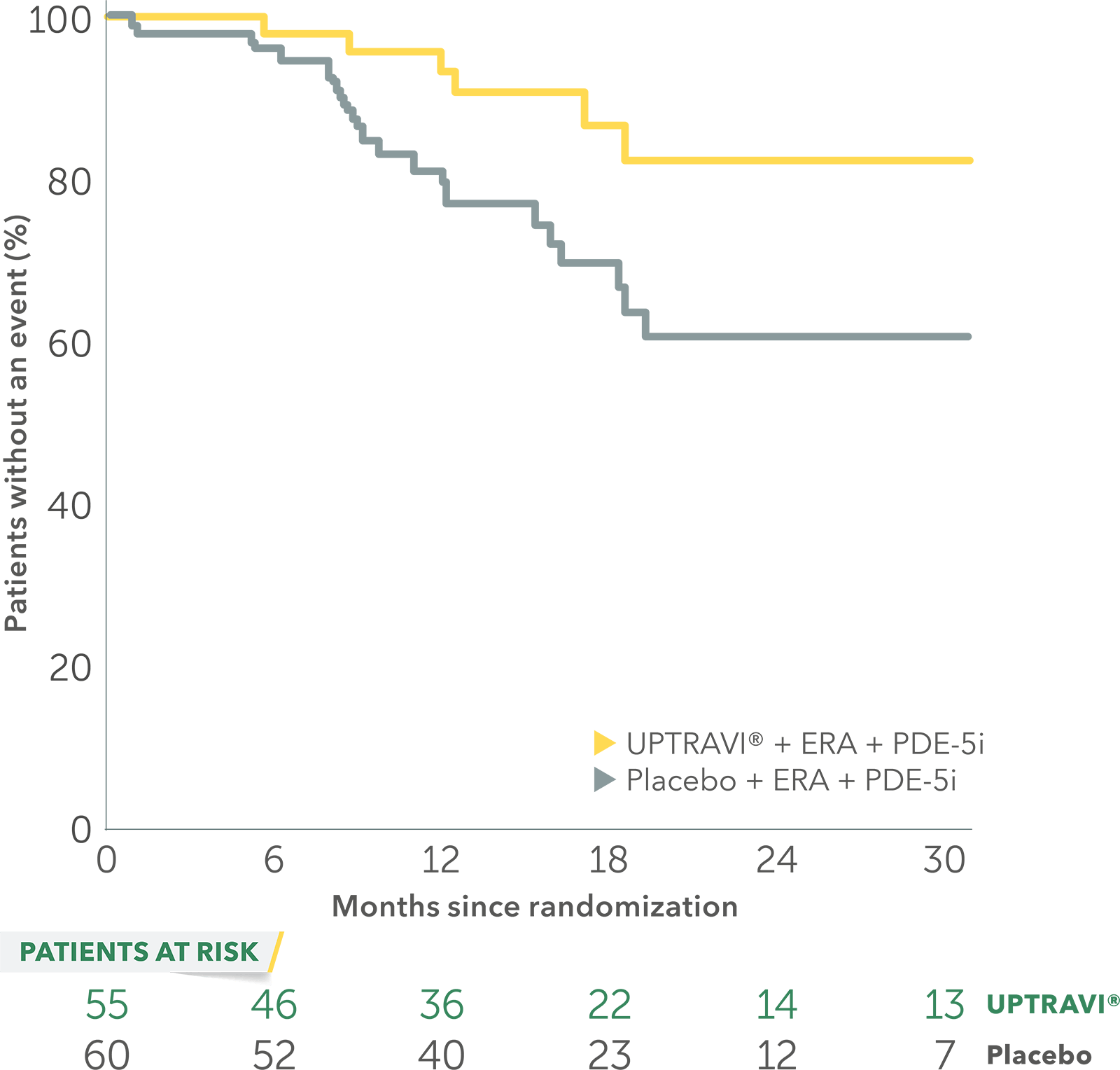
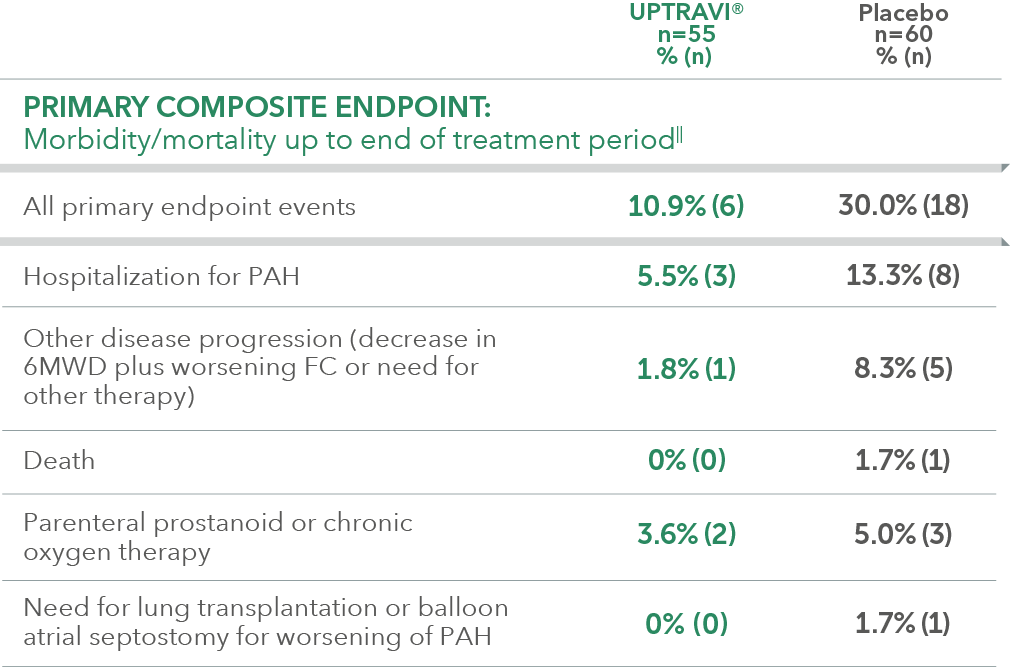
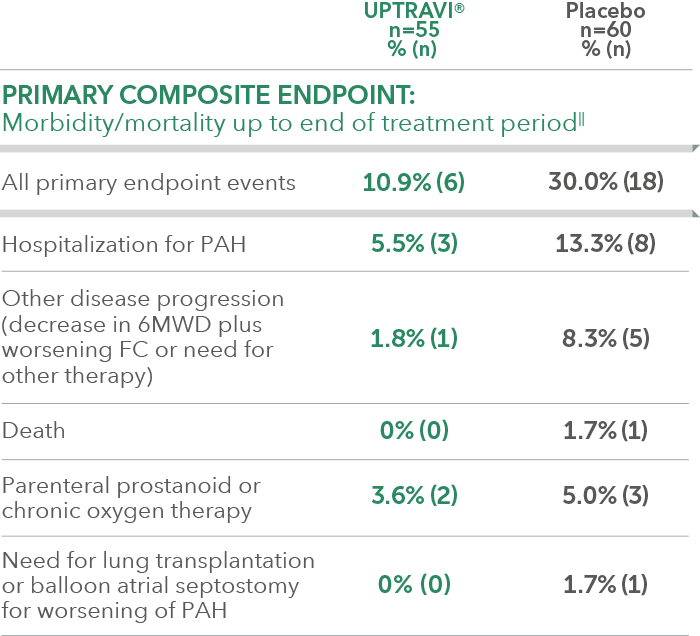
In an exploratory post hoc subgroup analysis, UPTRAVI® was associated with a: 64% RELATIVE RISK REDUCTION OF DISEASE PROGRESSION IN FC II PATIENTS ALREADY RECEIVING ERA + PDE-5i VS PLACEBO3*
Time to first disease progression event in FC II patients
receiving ERA + PDE-5i at baseline

Baseline patient characteristics
- 10% (n=115) of all patients in GRIPHON were FC II and receiving an ERA and a PDE-5i at baseline
- Etiology in FC II triple-combination subpopulation: IPAH/HPAH (70%), PAH-CTD (18%), PAH-CHD (6%), other (6%)‡
- Time from diagnosis in FC II patients: UPTRAVI® (4.3 years), placebo (3.6 years)
Summary of primary endpoint events in FC II patients receiving ERA + PDE-5i at baseline
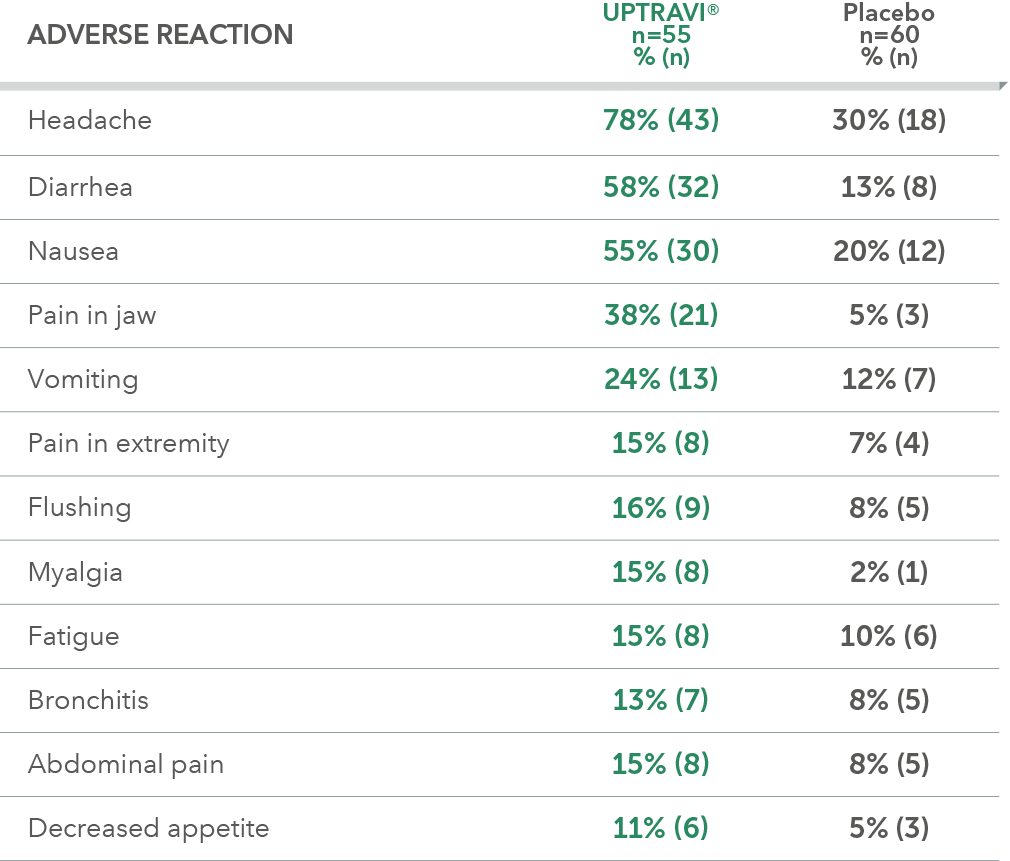
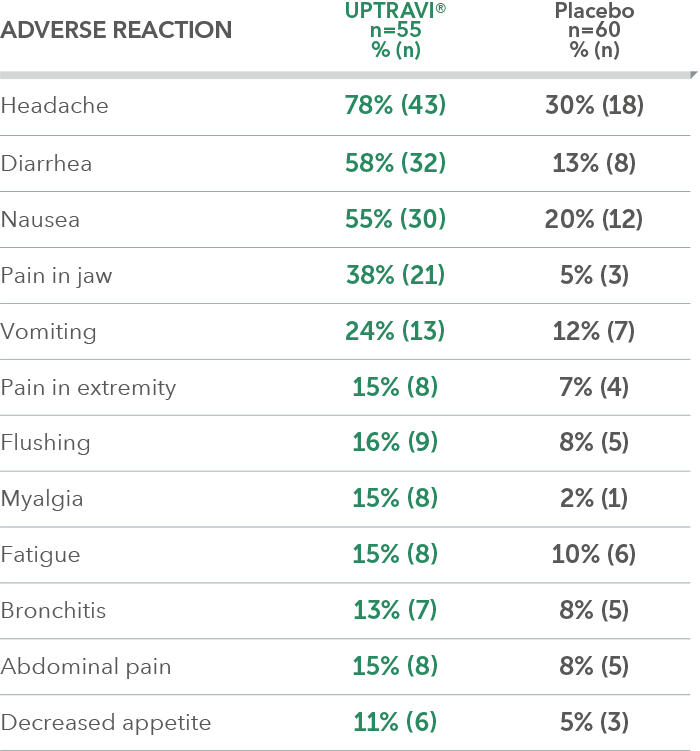
Adverse reactions in FC II triple-combination subgroup occurring more frequently with UPTRAVI® compared with placebo by ≥3%
2022 ESC/ERS Guidelines: UPTRAVI® is the only prostacyclin pathway therapy to receive the highest-class recommendation (Class I, Level B)¶ for sequential triple- and dual-combination therapy4
Patients receiving an ERA and a PDE-5i at baseline were a prespecified subgroup for evaluation of the GRIPHON primary endpoint; however, the more detailed analyses described on this page are exploratory and post hoc. Sample size should be considered and results should be interpreted with caution.
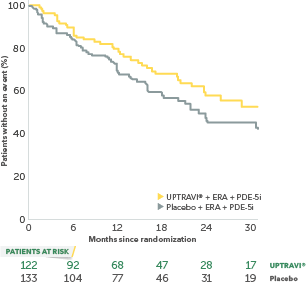
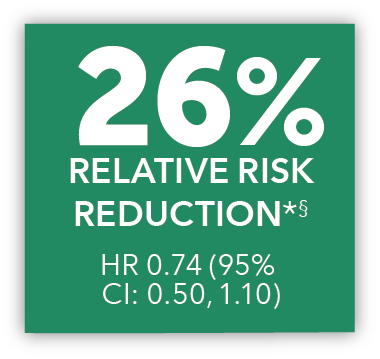
In an exploratory post hoc subgroup analysis, UPTRAVI® was associated with a: 26% RELATIVE RISK REDUCTION OF DISEASE PROGRESSION IN FC III PATIENTS ALREADY RECEIVING ERA + PDE-5i VS PLACEBO3*
Time to first disease progression event in FC III patients
receiving ERA + PDE-5i at baseline
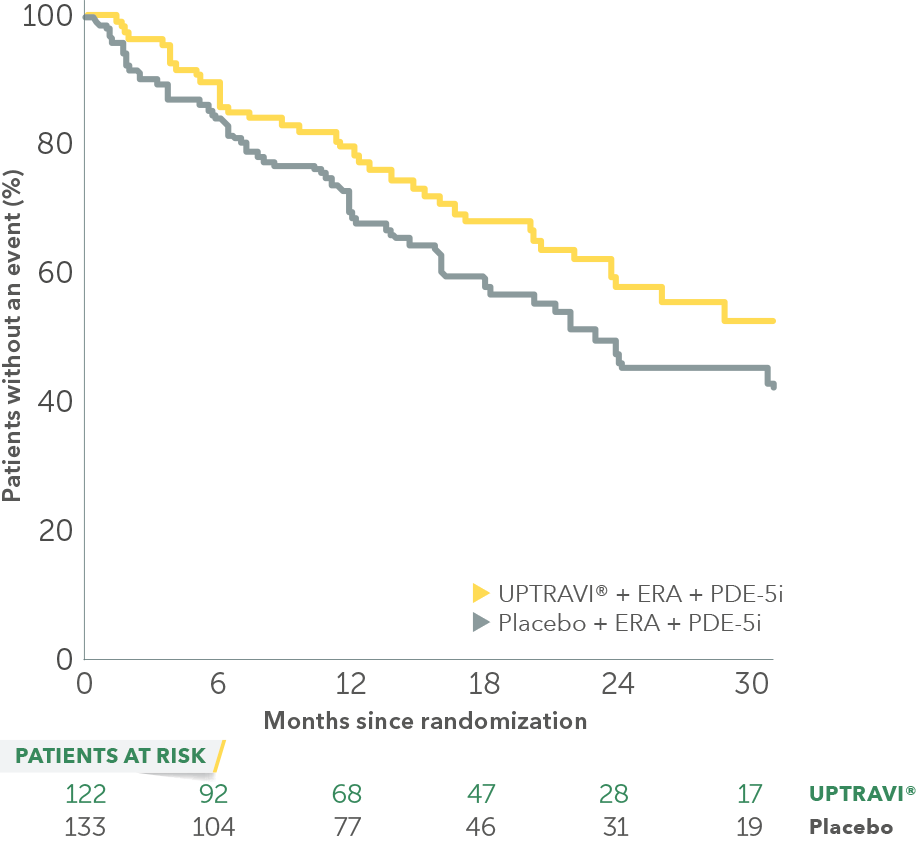
Baseline patient characteristics
- 22% (n=255) of all patients in GRIPHON were FC III and receiving an ERA and a PDE-5i at baseline
- Etiology in FC III triple-combination subpopulation: IPAH/HPAH (61%), PAH-CTD (29%), PAH-CHD (5%), other (4%)‡
- Time from diagnosis in FC III patients: UPTRAVI® (3.9 years), placebo (3.6 years)
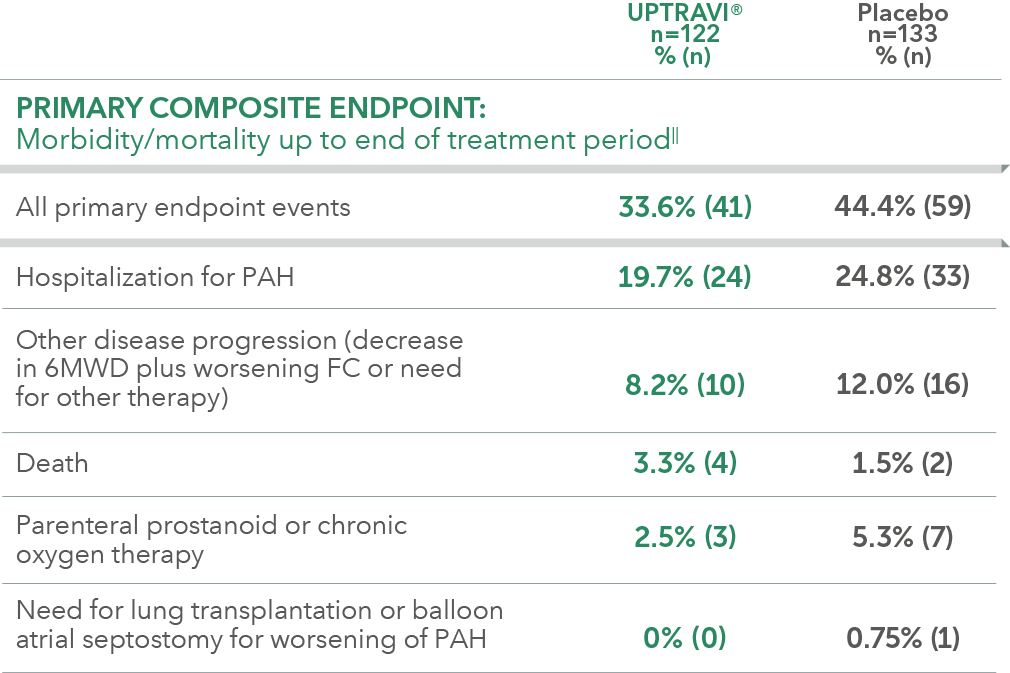
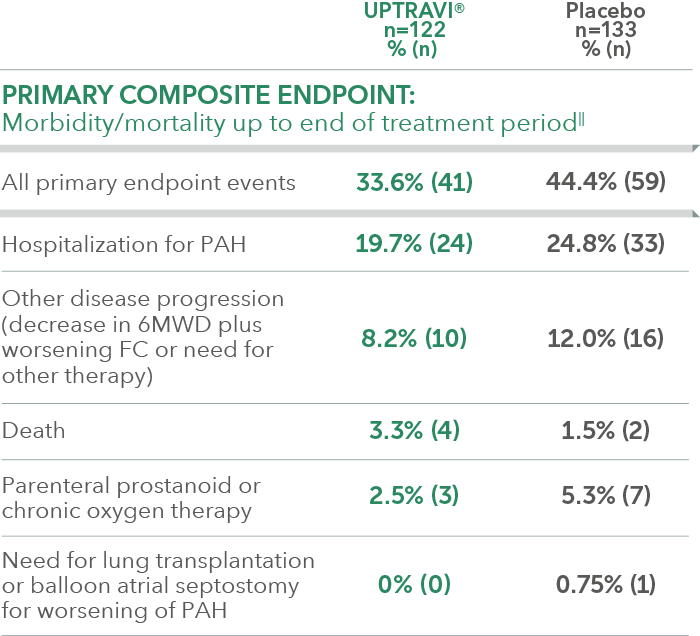
Summary of primary endpoint events in FC III patients receiving ERA + PDE-5i at baseline
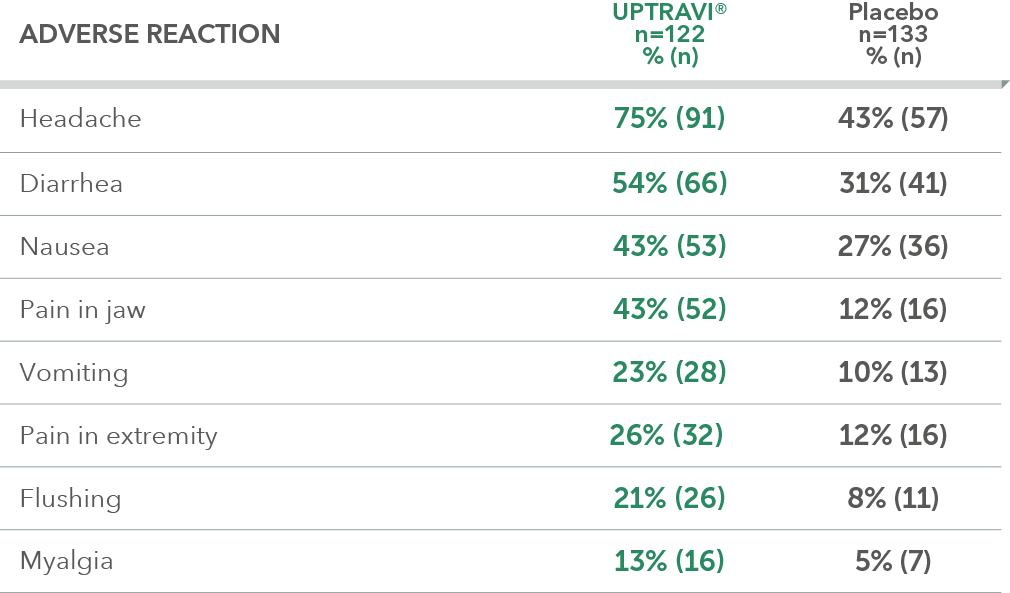
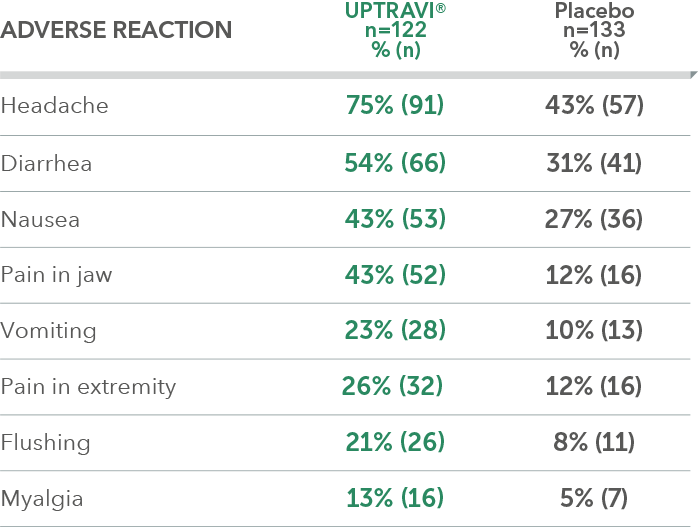
Adverse reactions in FC III triple-combination subgroup occurring more frequently with UPTRAVI® compared with placebo by ≥3%
2022 ESC/ERS Guidelines: UPTRAVI® is the only prostacyclin pathway therapy to receive the highest-class recommendation (Class I, Level B)¶ for sequential triple- and dual-combination therapy4
Patients receiving an ERA and a PDE-5i at baseline were a prespecified subgroup for evaluation of the GRIPHON primary endpoint; however, the more detailed analyses described on this page are exploratory and post hoc. Sample size should be considered and results should be interpreted with caution.