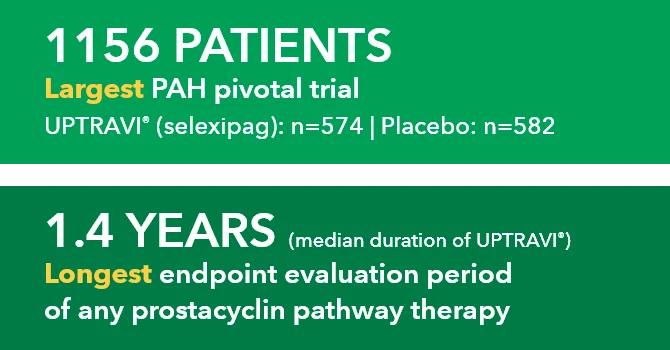
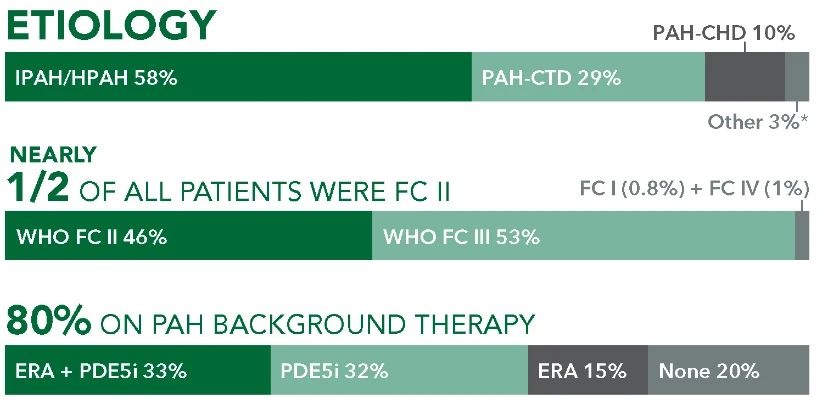
Uninterrupted treatment is key in managing pulmonary arterial hypertension (PAH, WHO Group I), a progressive disease.1
UPTRAVI® IV Is for the Treatment of PAH (WHO Group I) in FC II-III Patients Who Are Temporarily Unable to Take Oral Therapy2
- Avoid treatment disruptions when your patients are unable to take oral therapy by temporarily transitioning them from their current oral dose to IV treatment and returning to UPTRAVI® Tablets when possible2,3

Remain confident that your patients can receive UPTRAVI® treatment at home, in the hospital, and after discharge
- Expected to maintain similar UPTRAVI® efficacy and safety, with the exception of infusion-site reactions, when temporarily using UPTRAVI® IV in the hospital2,3
- Bypass re-titration when transitioning between UPTRAVI® IV and UPTRAVI® Tablets2
- UPTRAVI® Tablets (twice daily)*
- 200 mcg
- 400 mcg
- 600 mcg
- 800 mcg
- 1000 mcg
- 1200 mcg
- 1400 mcg
- 1600 mcg
- UPTRAVI® IV (twice daily infusions)
- 225 mcg
- 450 mcg
- 675 mcg
- 900 mcg
- 1125 mcg
- 1350 mcg
- 1575 mcg
- 1800 mcg
Administer UPTRAVI® IV twice daily by intravenous infusion at a dose that corresponds to the patient’s current dose of UPTRAVI® Tablets.
Please see complete UPTRAVI® IV dosing and administration instructions as listed in the full Prescribing Information.
UPTRAVI® IV INSTRUCTIONS FOR USE VIDEO
Watch the video below to learn more about storage, reconstitution, dilution, and administration of UPTRAVI® IV.
Select a Chapter
Downloadable Resources
UPTRAVI® IV Instructions for Use Guide
Use this guide to learn about storage, reconstitution, dilution, and administration of UPTRAVI® IV.
Temporarily Switching to UPTRAVI® IV From UPTRAVI® Tablets Was Well Tolerated With Comparable Exposure to the Active Metabolite
Study design3
- Phase 3, prospective, multicenter, open-label, single-sequence crossover study
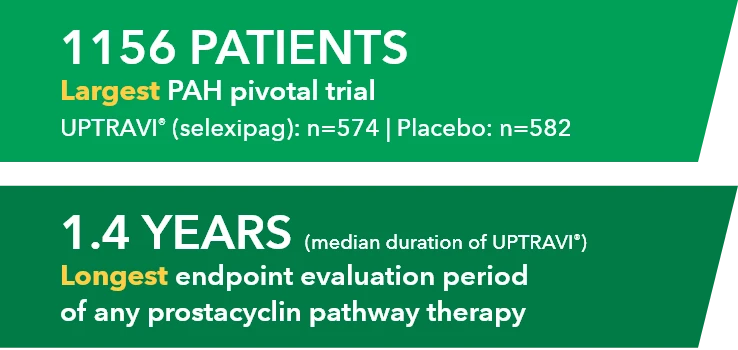
- Twenty patients with PAH in FC I-III† receiving a stable dose of UPTRAVI® Tablets for ≥28 days prior to enrollment were included
- Patients were monitored to assess the safety, tolerability, and pharmacokinetics (including exposure to the active metabolite ACT-333679) of switching from a stable dose of UPTRAVI® Tablets to a corresponding dose of UPTRAVI® IV and back to UPTRAVI® Tablets
- The dosing regimen for UPTRAVI® IV was selected to help patients achieve similar exposure to the active metabolite when compared with UPTRAVI® Tablets
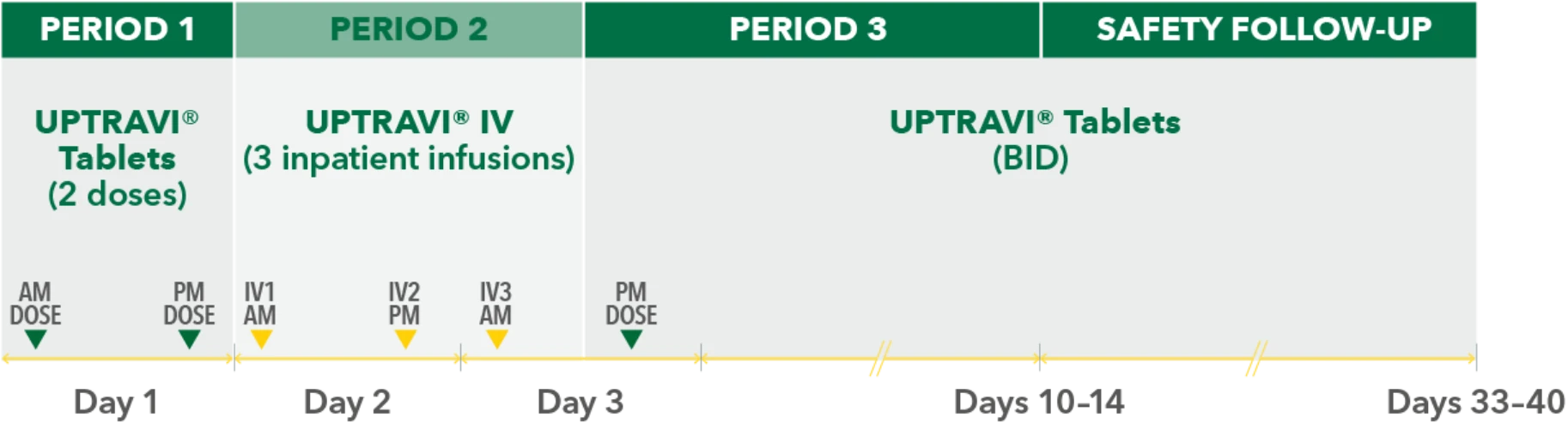
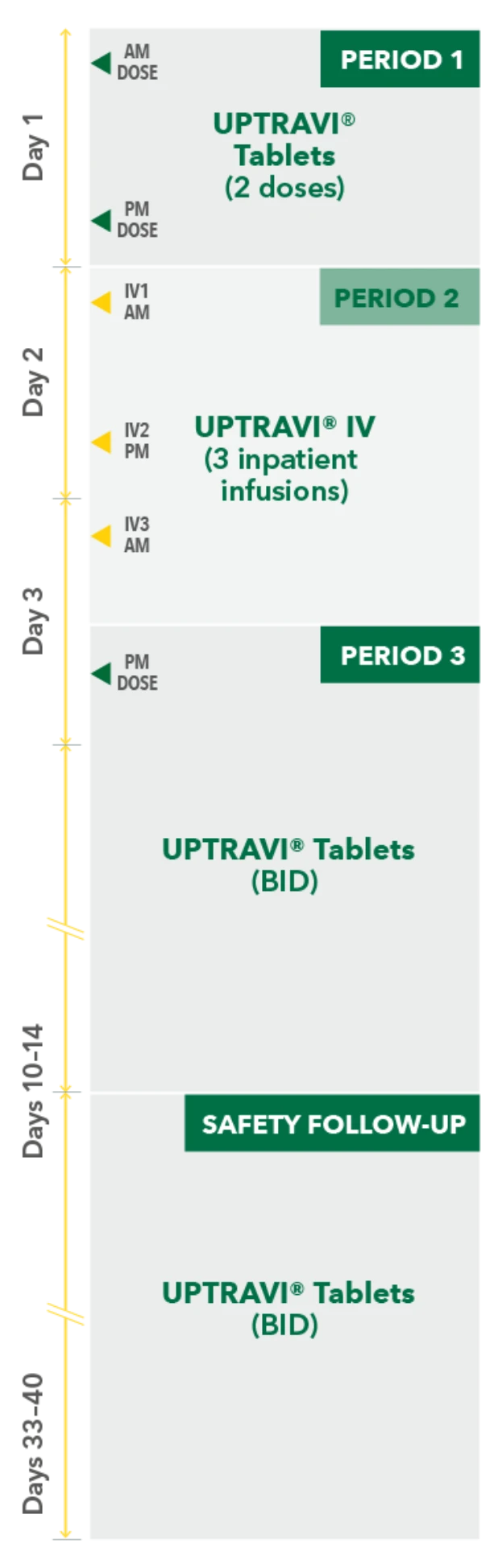
PERIOD 1
UPTRAVI® Tablets (2 doses)
PM DOSE
PERIOD 2
UPTRAVI® IV (3 inpatient infusions)
PERIOD 3
AM DOSE
SAFETY FOLLOW-UP
UPTRAVI® Tablets (BID)
IV1 AM
IV2 PM
IV3 AM
PM DOSE
Day 1
Day 2
Day 3
Days 10-14
Days 10-14
Baseline patient characteristics (N=20)3
- Mean age: 57 years
- Female: 80%
- Etiology: idiopathic or heritable PAH (70%), PAH-CTD (20%), PAH-CHD (5%), PoPH (5%)
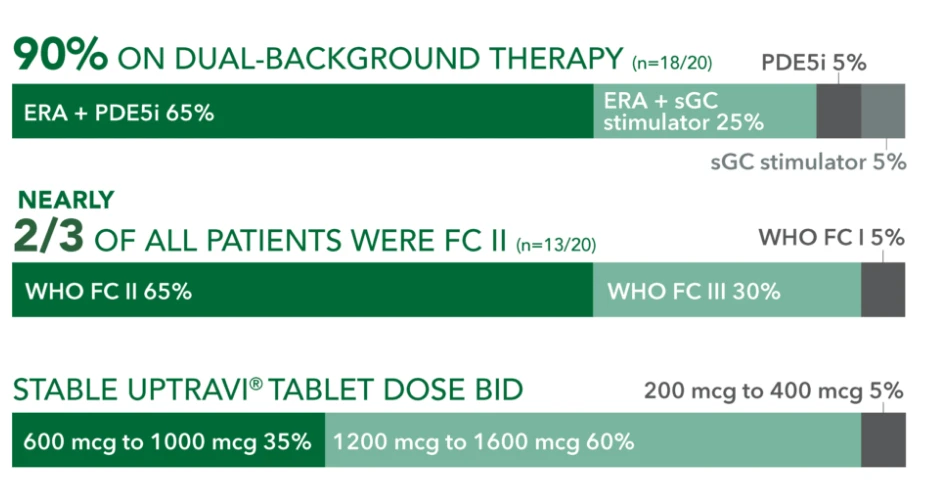
90% ON DUAL-BACKGROUND THERAPY (r=18/20)
ERA + sGC
stimulator 25%
sGC stimulator 5%
WHO FC 15%
WHO FC III 30%
PDE5i 5%
NEARLY 2/3 OF ALL PATIENTS WERE FC II (n=13/20) WHO FC II 65%
STABLE UPTRAVI® TABLET DOSE BID 600 mcg to 1000 mcg 35%
200 mcg to 400 mcg 5%
1200 mcg to 1600 mcg 60%
Results2,3
Comparable exposure to the active metabolite following UPTRAVI® Tablets and UPTRAVI® IV administration
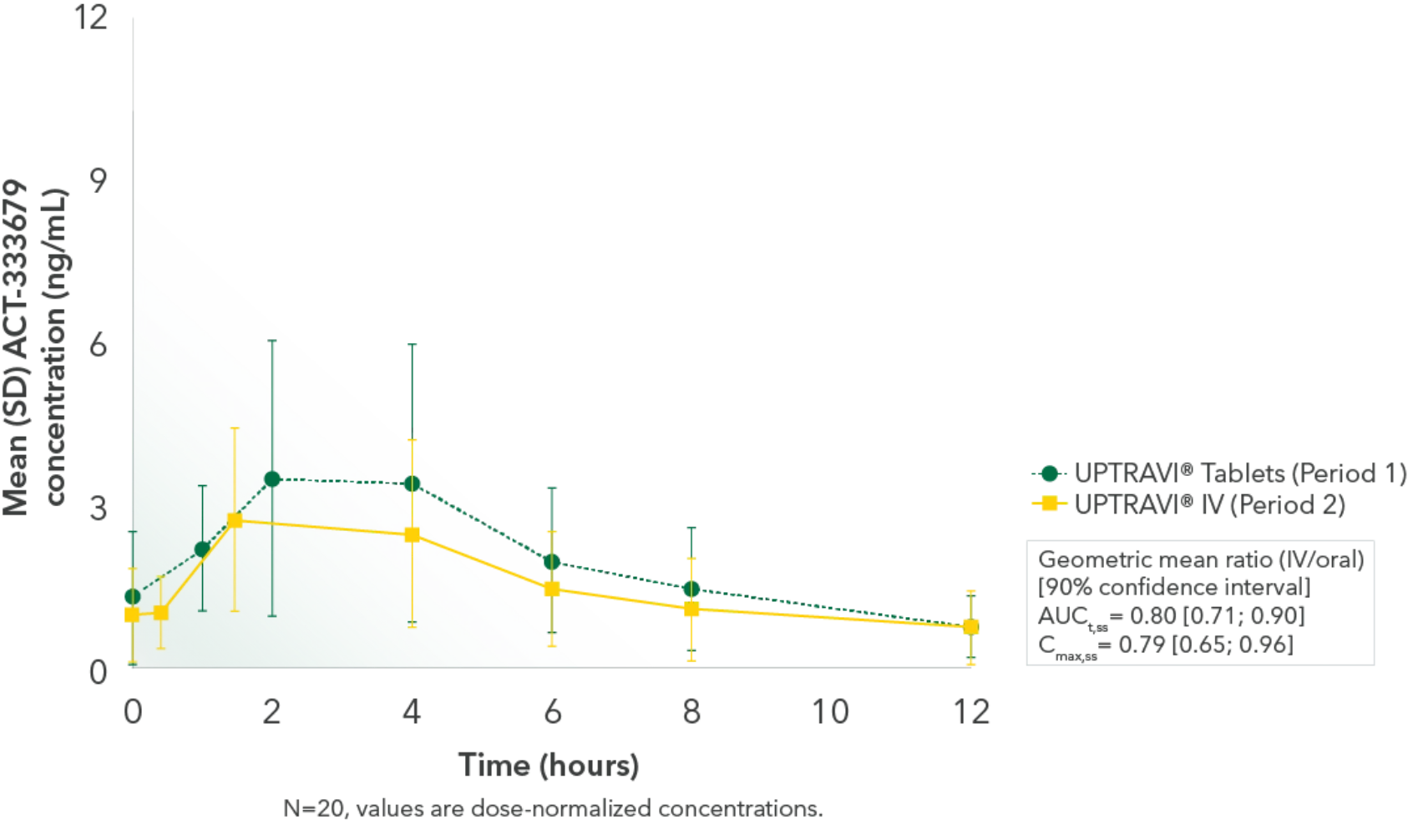
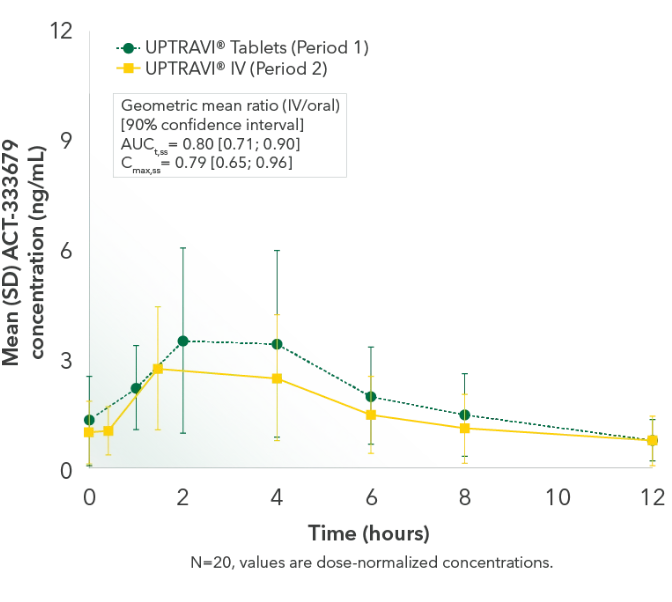
Time to the maximum concentration of the active metabolite was comparable
Safety2,3
- The transition from UPTRAVI® Tablets to UPTRAVI® IV and back was well tolerated: Patient tolerability was similar between UPTRAVI® IV and Tablets
- 10% of patients reported infusion-site reactions during UPTRAVI® IV administration, including infusion-site erythema/redness, pain, and swelling