Objective
Compare the effects of oral treprostinil with UPTRAVI® on PAH-related hospitalization among patients with PAH based on a retrospective claims database analysis.
Methods
Retrospective claims analysis of the Optum® Clinformatics® Data Mart database containing both medical and pharmacy administrative claims for privately insured US patients.*
- A multivariable Cox hazard regression model was used to compare time to first PAH-related hospitalization for oral treprostinil vs UPTRAVI®, adjusting for age, gender, geographic region, insurance type, Charlson comorbidity score, baseline PAH medication, certain comorbidities, and baseline inpatient and outpatient visits
Outcome measures
The primary outcome was PAH-related hospitalization† with a PH diagnosis code at any position in the medical claim.
Study design
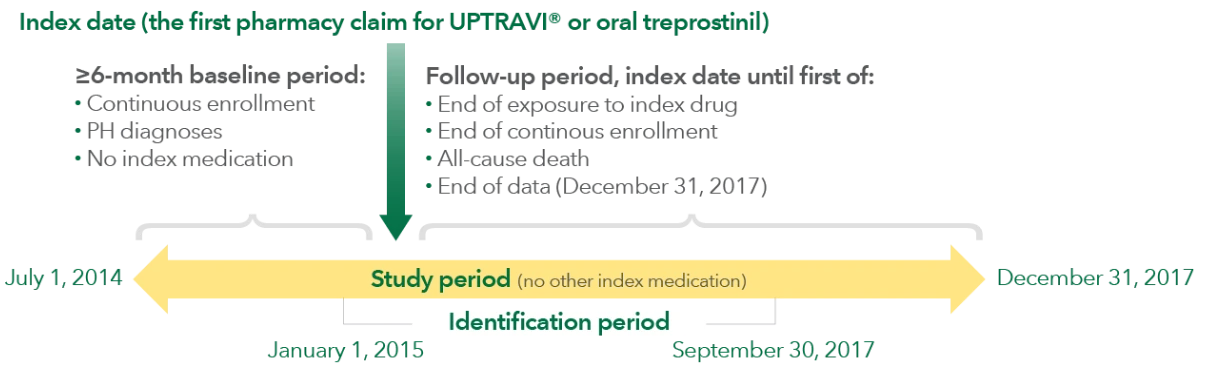
Patient selection
Inclusion criteria included the following:
- Age ≥18 years
- Continuous enrollment with ≥1 medical claim with ICD diagnosis code for PH‡ in the baseline period of ≥6 months prior to the index date
- New initiation of UPTRAVI® or oral treprostinil; could not have prior oral PPA treatment in the 6 months before index date
- Could not switch oral PPA during the study period
- Continuous treatment based on prescription claim with subsequent claim ≤45 days following the end of the days’ supply of the previous claim
Study limitations and considerations
- This retrospective claims analysis compared risk of PAH-related hospitalization and does not assess or compare product safety or efficacy. There have been no head-to-head clinical trials comparing the efficacy or safety of UPTRAVI® and oral treprostinil. No direct evaluation of comparative clinical profiles can be made
- Due to the limitations, it is not possible to draw firm conclusions regarding causality of the association found in this study. Controlled studies are recommended to confirm findings
- Results should be considered as hypothesis generating and should not be taken as the basis for influencing clinical practice
- This research was sponsored by Actelion Pharmaceuticals US, Inc., a Johnson & Johnson company. The sponsor was involved in the data analysis and interpretation, funded medical writing and editorial support to the authors, and was involved in the decision to publish the finished manuscript
- Prevalence of comorbidities and PAH diagnosis were obtained from administrative claims data that have not been clinically validated. Administrative claims do not provide information on whether the prescriptions were filled and taken as prescribed
- There are no unique ICD-9-CM or ICD-10-CM codes for PAH. Both coding systems have a diagnostic code for primary PH, which corresponds to idiopathic PAH in the current PAH classification. However, they have no codes that differentiate other PAH subgroups from non-PAH forms of PH. Since patients were identified based on the diagnosis code for PH, there may be an unknown percentage of non-PAH patients included in the study
- Patients were excluded from switching between oral treprostinil and UPTRAVI®
- This study included US patients who are privately insured, thus generalizability of results to other populations such as patients with different coverage, to the US population as a whole, or to populations residing out of the US is not clear
- Despite covariate adjustments in the multivariate Cox proportional hazard model, unmeasured confounders, such as WHO FC, exercise capacity, right ventricular function and structure, hemodynamics, brain natriuretic peptide, and other biomarkers, may impact the study results
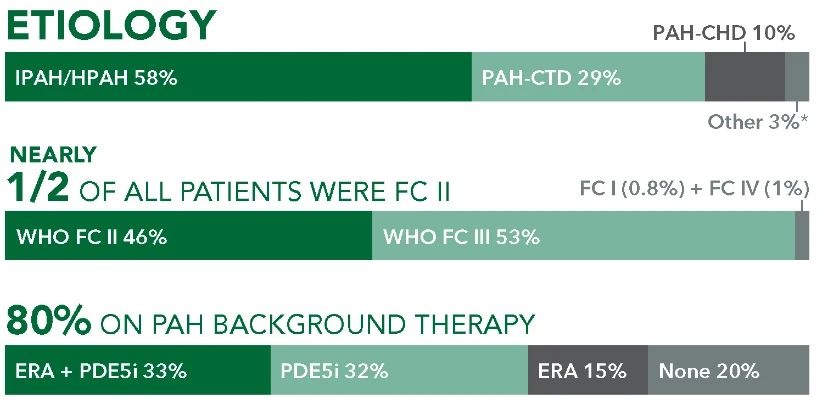
Baseline patient characteristics1
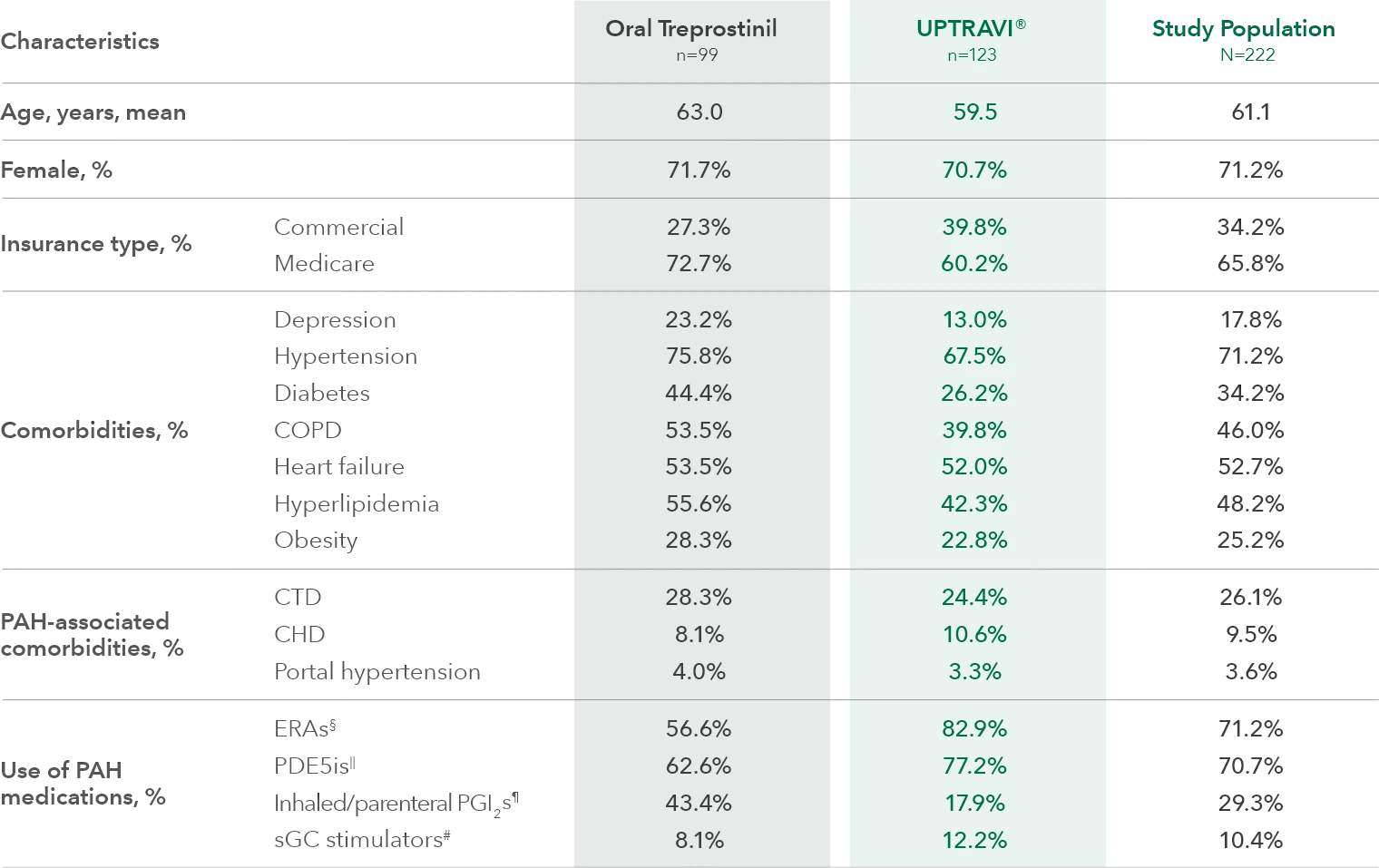
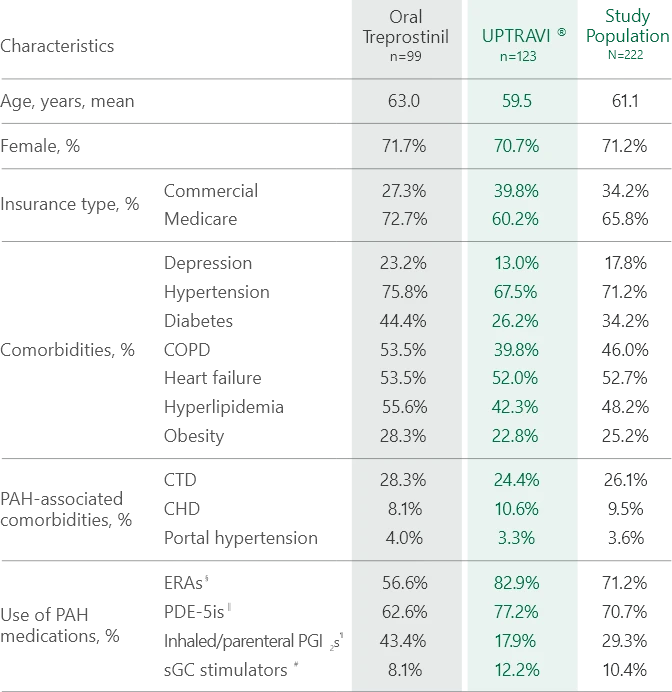
First PAH-related hospitalization risk analysis1
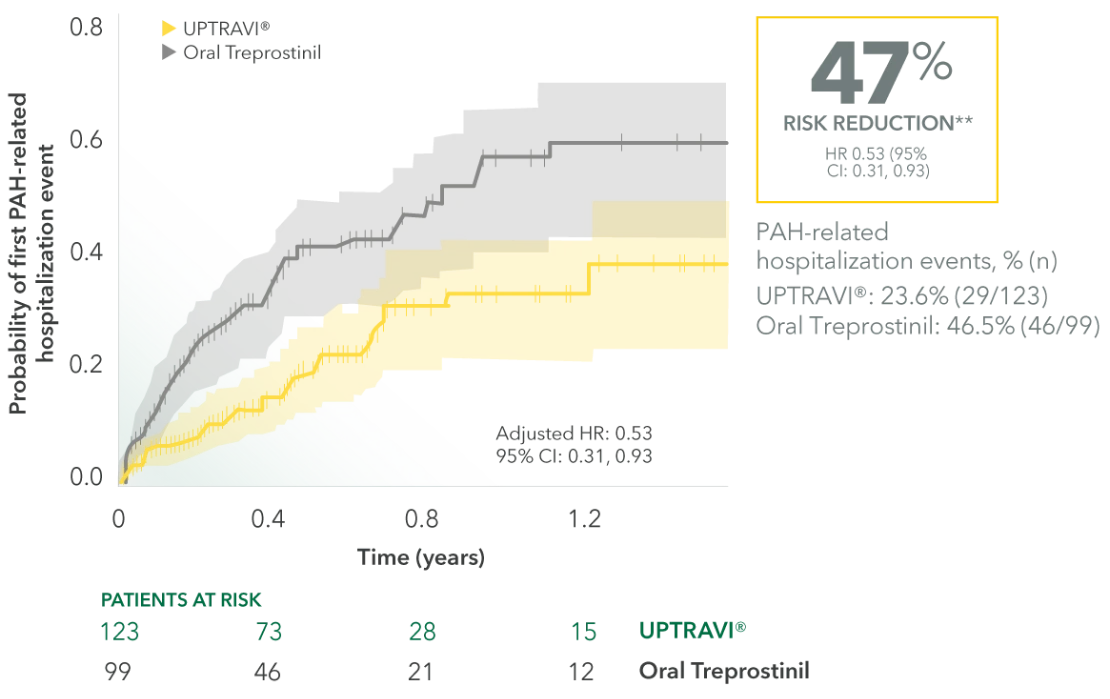

Reference group: oral treprostinil; the Cox proportional hazards models were adjusted for age, gender, geographic region, insurance type, Charlson comorbidity score, PAH medication, PAH-associated comorbidities, other selected comorbidities, and baseline hospitalization and outpatient visits.
- This retrospective claims analysis compared risk of PAH-related hospitalization and does not assess or compare product safety or efficacy. Prevalence of comorbidities and PAH diagnosis were obtained from administrative claims data that have not been clinically validated
- There have been no head-to-head clinical trials comparing the efficacy or safety of UPTRAVI® and oral treprostinil. No direct evaluation of comparative clinical profiles can be made
- Results should be considered as hypothesis generating and should not be taken as the basis for influencing clinical practice