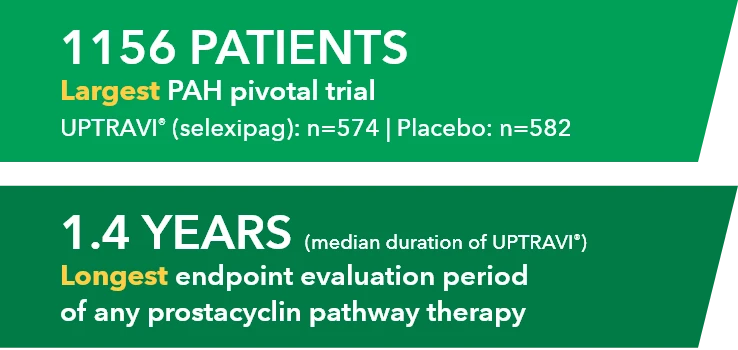
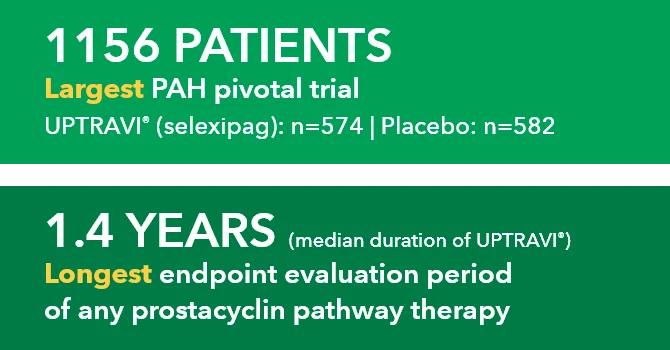
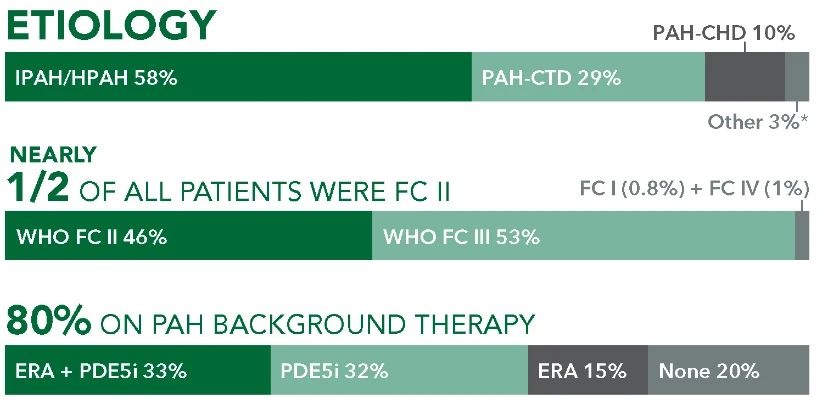
Baseline patient characteristics1*
Patients with PAH-CTD in GRIPHON
29% (n=334/1156)
Subtypes
- 51%
PAH-SSc
(n=170/334) - 24.5%
PAH-SLE
(n=82/334) - 24.5%
PAH-MCTD/CTD-Other
(n=82/334)†
FC II
46%
(n=154/334)FC III
53%
PAH-SLE
(n=176/334)FC I OR IV
1%
(n=4/334)
Background therapy
- 28%
PDE5i
(n=82/334) - 23%
None
(n=78/334) - 20%
ERA
(n=66/334) - 29%
ERA + PDE5i
(n=96/334)
Time to first disease progression event in patients with PAH-CTD (UPTRAVI® vs placebo)1
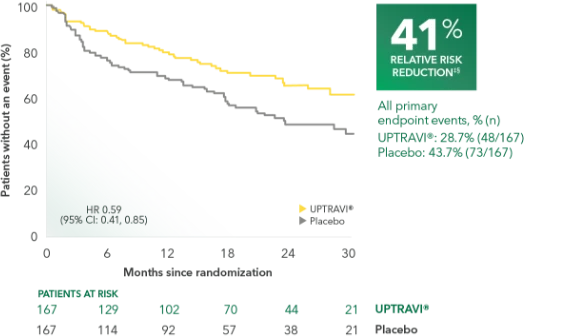
Summary of primary endpoint events in patients with PAH-CTD1
| UPTRAVI® n=77 % (n) | Placebo n=197 % (n) | |
|---|---|---|
| PRIMARY COMPOSITE ENDPOINT: Morbidity/mortality up to end of treatment period|| | ||
| All primary endpoint events | 28.7% (48) | 43.7% (73) |
| Hospitalization for PAH | 12.6% (21) | 21.6% (36) |
| Other disease progression (decrease in 6MWD plus worsening FC or need for other therapy) | 6.6% (11) | 17.4% (29) |
| Death | 7.2% (12) | 1.8% (3) |
| Parenteral prostanoid or chronic oxygen therapy | 1.8% (3) | 3.0% (5) |
| Need for lung transplantation or balloon atrial septostomy for worsening of PAH | 0.6% (1) | 0% (0) |
PAH-CTD was a prespecified subgroup for evaluation of the primary endpoint; however, the more detailed analyses described here are post hoc. Sample size should be considered and results should be interpreted with caution.
Adverse reactions in the PAH-CTD subpopulation occurring more frequently with UPTRAVI® compared with placebo by ≥3%1
| ADVERSE REACTION | UPTRAVI® n=167 % (n) | Placebo n=165 % (n) |
|---|---|---|
| Headache | 62% (104) | 36% (60) |
| Diarrhea | 40% (67) | 26% (42) |
| Nausea | 37% (62) | 25% (41) |
| Dizziness | 21% (35) | 18% (30) |
| Vomiting | 20% (34) | 6% (10) |
| Pain in extremity | 19% (31) | 5% (8) |
| Pain in jaw | 14% (24) | 7% (11) |
| Myalgia | 13% (21) | 6% (10) |
| Arthalgia | 11% (19) | 7% (12) |
| Nasopharyngitis | 11% (19) | 7% (12) |
| Flushing | 11% (19) | 5% (8) |
| Decreased appetite | 10% (17) | 6% (9) |
Baseline patient characteristics1*
Of the 334 patients with PAH-CTD in GRIPHON,
Patients with PAH-SSc
51% (n=170/334)
FC II
34%
(n=57/170)FC III
65%
PAH-SLE
(n=110/170)FC I OR IV
2%
(n=3/170)
Background therapy
- 23%
PDE5i
(n=39/170) - 22%
None
(n=38/170) - 18%
ERA
(n=31/170) - 37%
ERA + PDE5i
(n=62/170)
Time to first disease progression event in patients with PAH-SSc (UPTRAVI® vs placebo)1
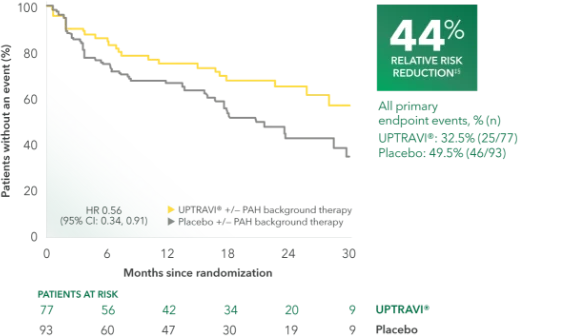
Summary of primary endpoint events in patients with PAH-SSc1
| UPTRAVI® n=179 % (n) | Placebo n=197 % (n) | |
|---|---|---|
| PRIMARY COMPOSITE ENDPOINT: Morbidity/mortality up to end of treatment period|| | ||
| All primary endpoint events | 32.5% (25) | 49.5% (46) |
| Hospitalization for PAH | 13.0% (10) | 23.7% (22) |
| Other disease progression (decrease in 6MWD plus worsening FC or need for other therapy) | 10.4% (8) | 19.4% (18) |
| Death | 5.2% (4) | 1.1% (1) |
| Parenteral prostanoid or chronic oxygen therapy | 2.6% (2) | 5.4% (5) |
| Need for lung transplantation or balloon atrial septostomy for worsening of PAH | 1.3% (1) | 0% (0) |
This subgroup analysis was post hoc and exploratory in nature. Sample size should be considered and results should be interpreted with caution.
Adverse reactions in the PAH-SSc subpopulation occurring more frequently with UPTRAVI® compared with placebo by ≥3%1
| ADVERSE REACTION | UPTRAVI® n=77 % (n) | Placebo n=91‖ % (n) |
|---|---|---|
| Headache | 55% (42) | 34% (31) |
| Diarrhea | 46% (35) | 28% (25) |
| Nausea | 31% (24) | 26% (24) |
| Dizziness | 22% (17) | 18% (16) |
| Pain in extremity | 22% (17) | 4% (4) |
| Pain in jaw | 20% (15) | 8% (7) |
| Back pain | 14% (11) | 7% (6) |
| Myalgia | 12% (9) | 8% (7) |
| Urinary tract infection | 10% (8) | 7% (6) |
| Asthenia | 10% (8) | 4% (4) |