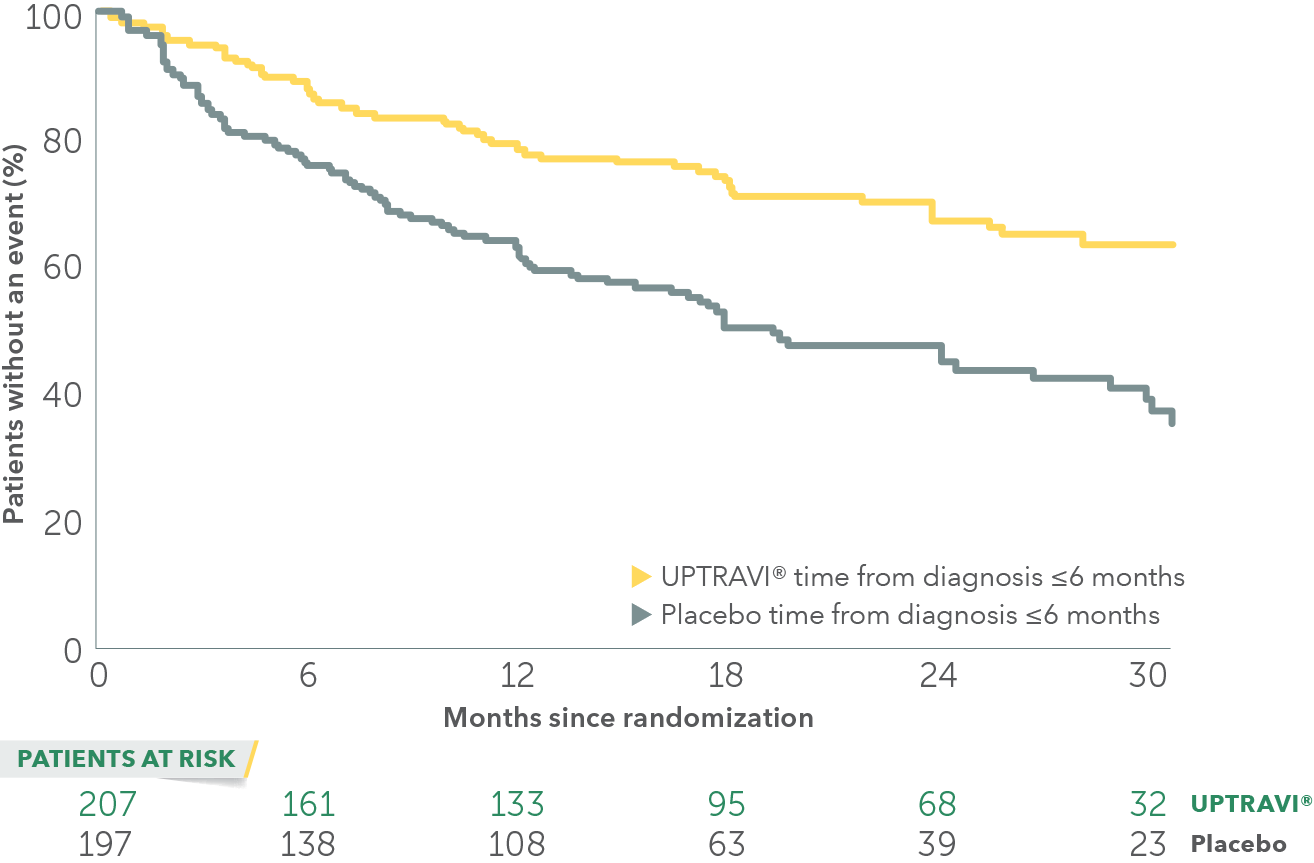
In an exploratory post hoc subgroup analysis, UPTRAVI® was associated with a: 55% RELATIVE RISK REDUCTION OF DISEASE PROGRESSION WHEN UPTRAVI® WAS USED WITHIN 6 MONTHS OF DIAGNOSIS VS PLACEBO1*
Time to first disease progression event in patients treated within 6 months of PAH diagnosis

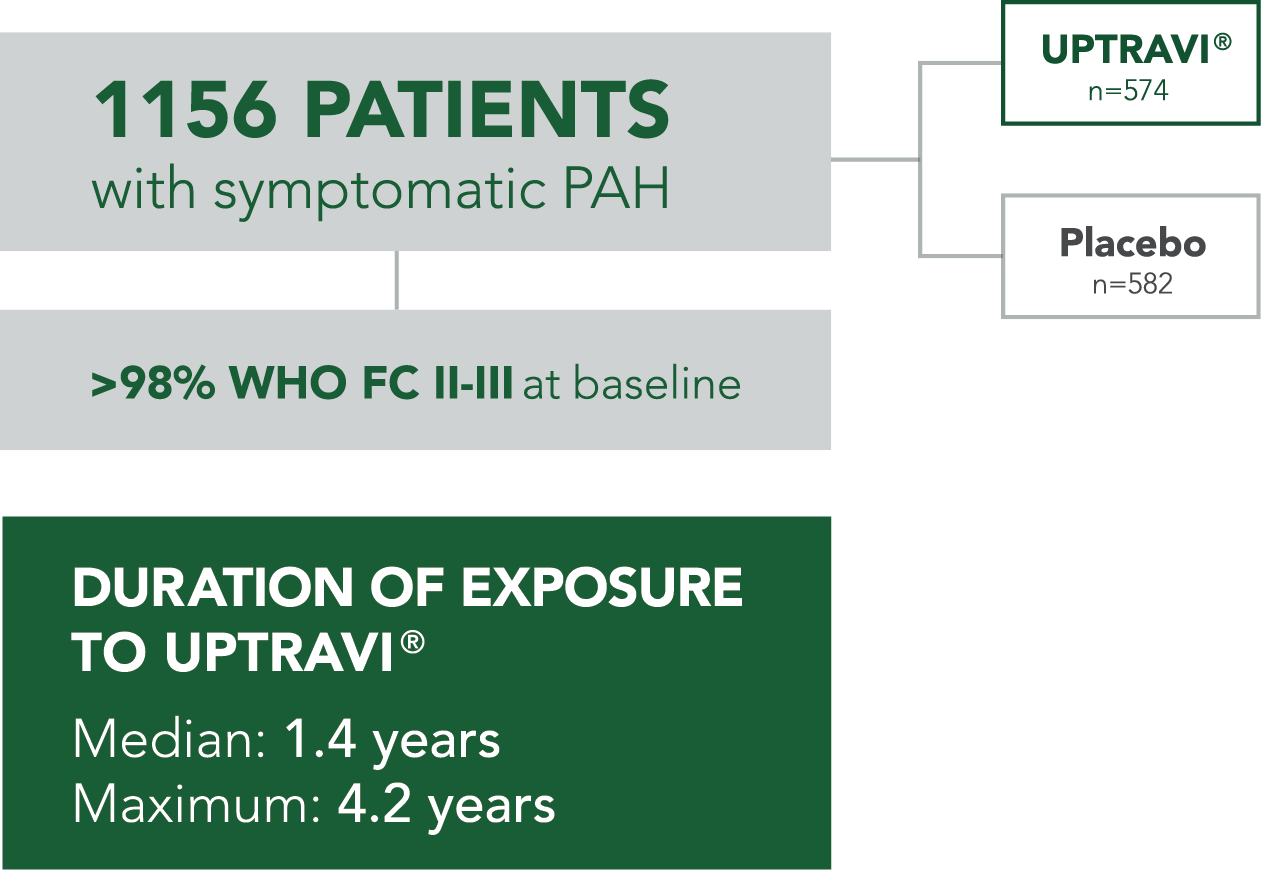
Baseline patient characteristics
- GRIPHON patients were categorized based on their time from diagnosis at baseline using a 6-month threshold:
- 35% (n=404) of all patients in GRIPHON were treated ≤6 months from diagnosis (52% FC II and 46% FC III)†
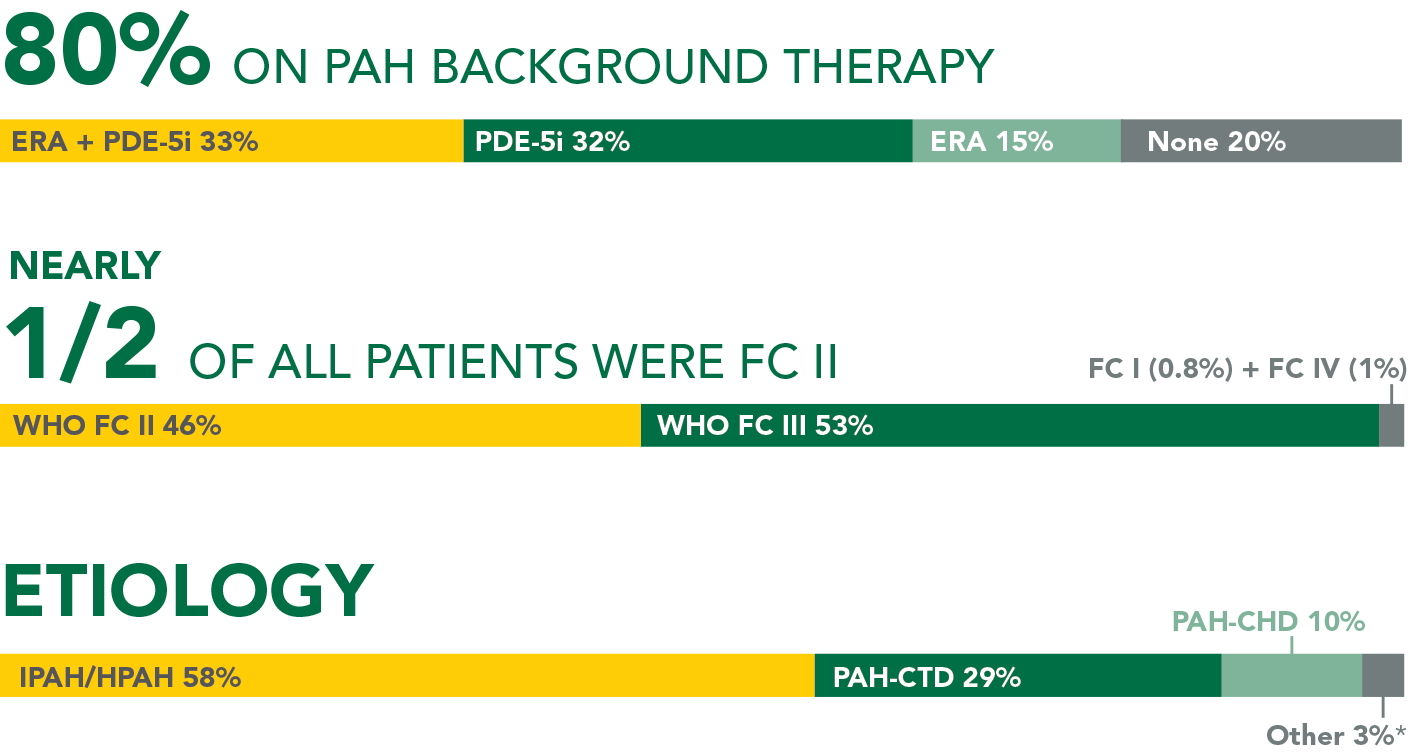
- 41% were receiving PDE-5i monotherapy, 39% were receiving no background PAH therapy, 10% were receiving ERA monotherapy, and 10% were receiving an ERA + PDE-5i at baseline
- Mean age: UPTRAVI® (44 years), placebo (44 years)
- FC II: UPTRAVI® (55%), placebo (49%)
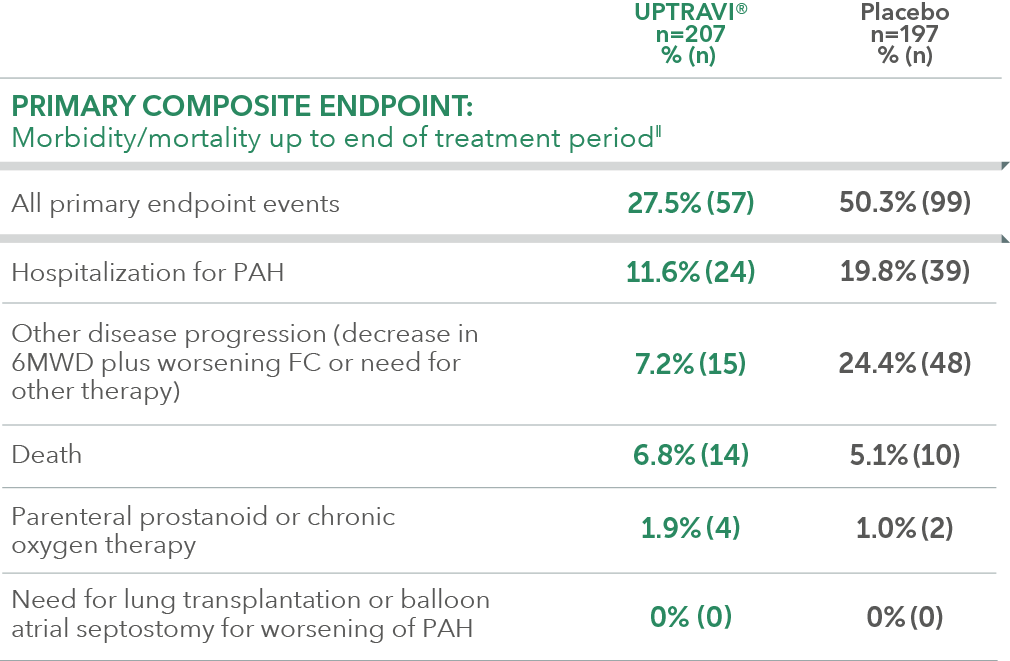
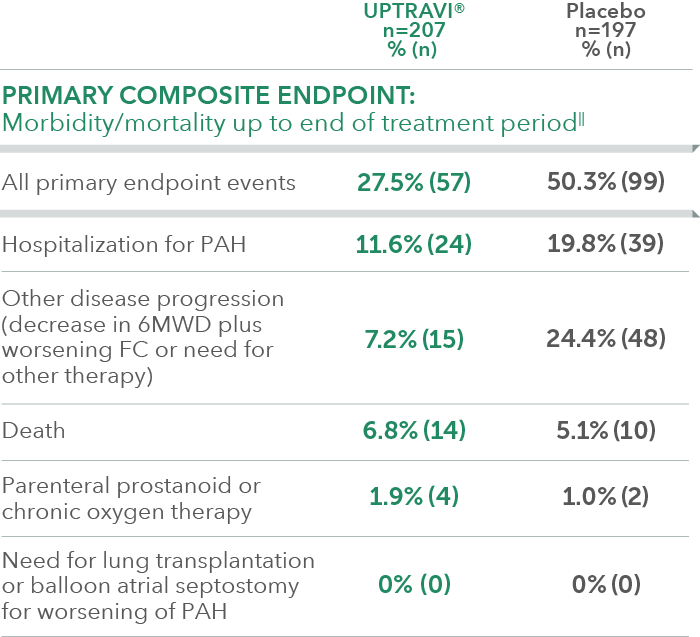
Summary of primary endpoint events in patients treated within 6 months of PAH diagnosis
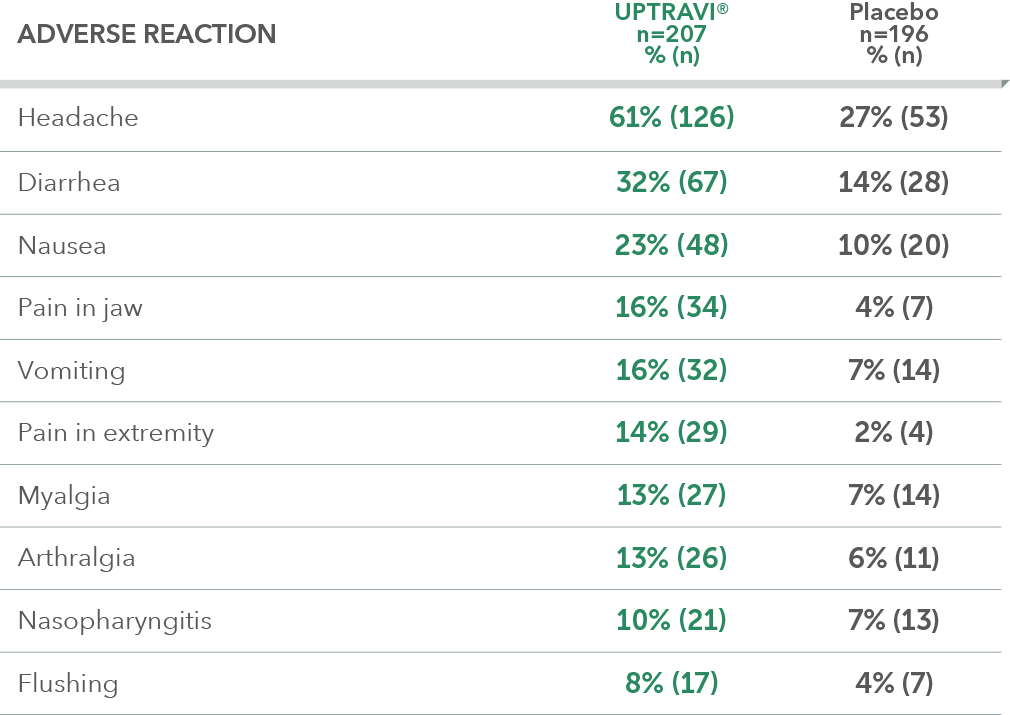
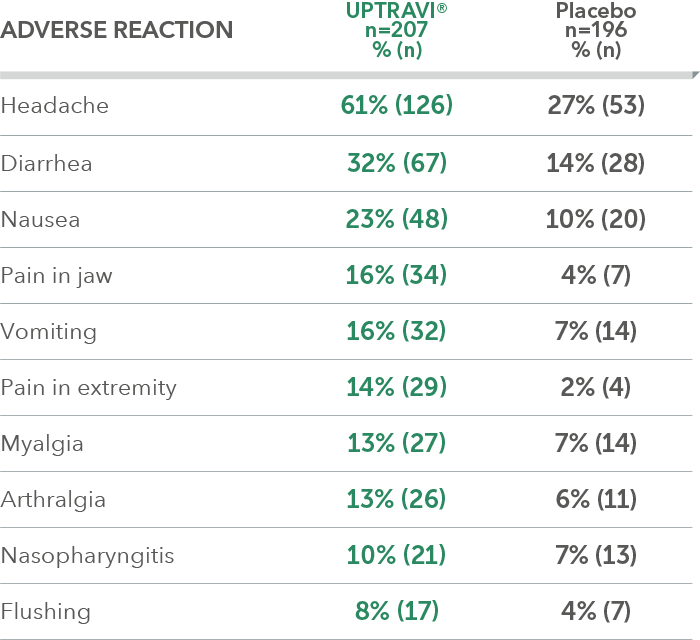
Adverse reactions in ≤6 months from diagnosis subgroup occurring more frequently with UPTRAVI® compared with placebo by ≥3%
The analysis described here is post hoc and exploratory. The subgroup was not prespecified for evaluation of the primary endpoint. Please note this analysis did not compare patients treated within 6 months of PAH diagnosis with patients treated after 6 months of PAH diagnosis. Sample size should be considered and results should be interpreted with caution.
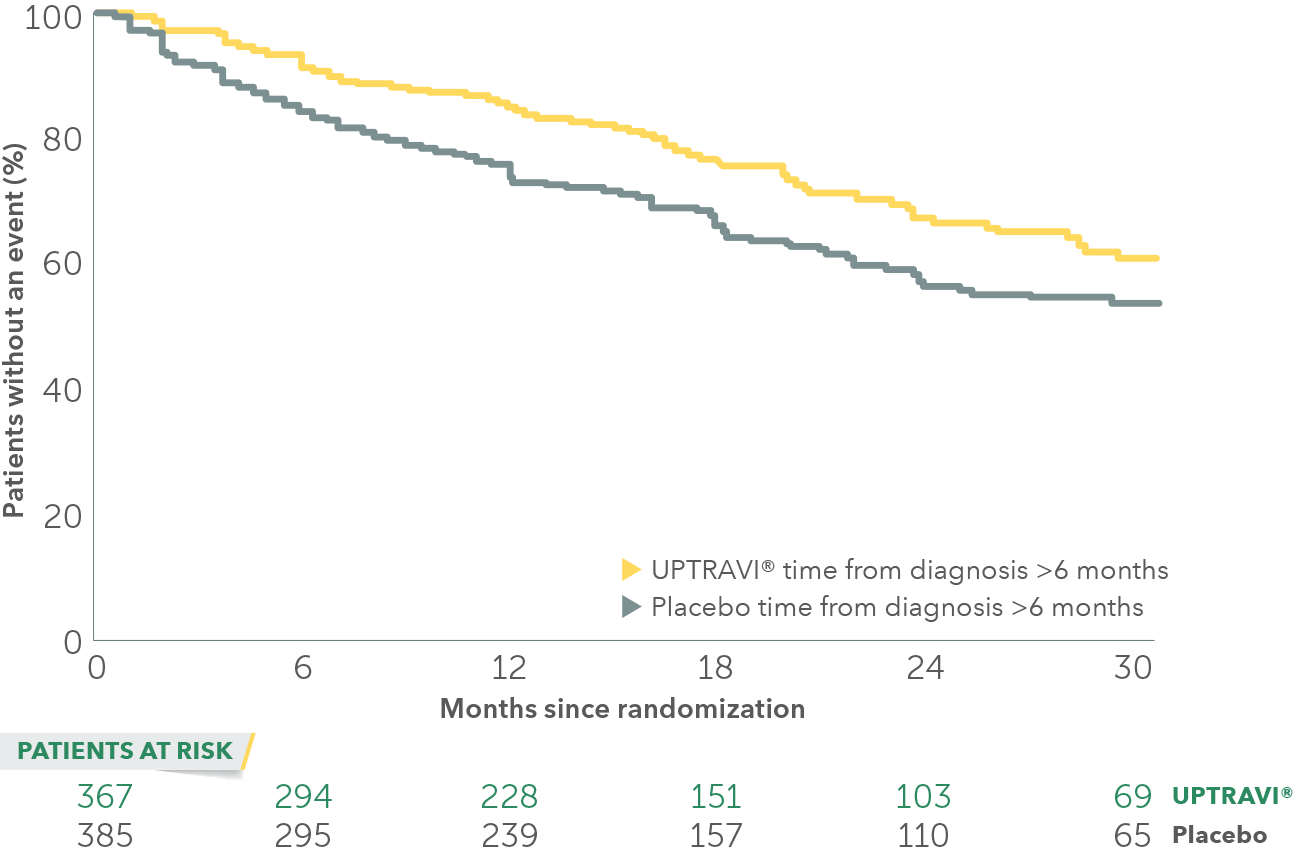
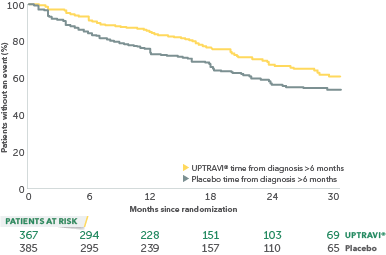
In an exploratory post hoc subgroup analysis, UPTRAVI® was associated with a: 26% RELATIVE RISK REDUCTION OF DISEASE PROGRESSION WHEN UPTRAVI® WAS USED AFTER 6 MONTHS OF DIAGNOSIS VS PLACEBO1*
Time to first disease progression event in patients treated after 6 months of PAH diagnosis

Baseline patient characteristics
- GRIPHON patients were categorized based on their time from diagnosis at baseline using a 6-month threshold:
- 65% (n=752) of all patients in GRIPHON were treated >6 months from diagnosis (56% FC III, 42% FC II)†
- 28% were receiving PDE-5i monotherapy, 11% were not receiving PAH background therapy, 17% were receiving ERA monotherapy, and 45% were receiving an ERA + PDE-5i at baseline
- Mean age: UPTRAVI® (50 years), placebo (50 years)
- FC II: UPTRAVI® (44%), placebo (41%)
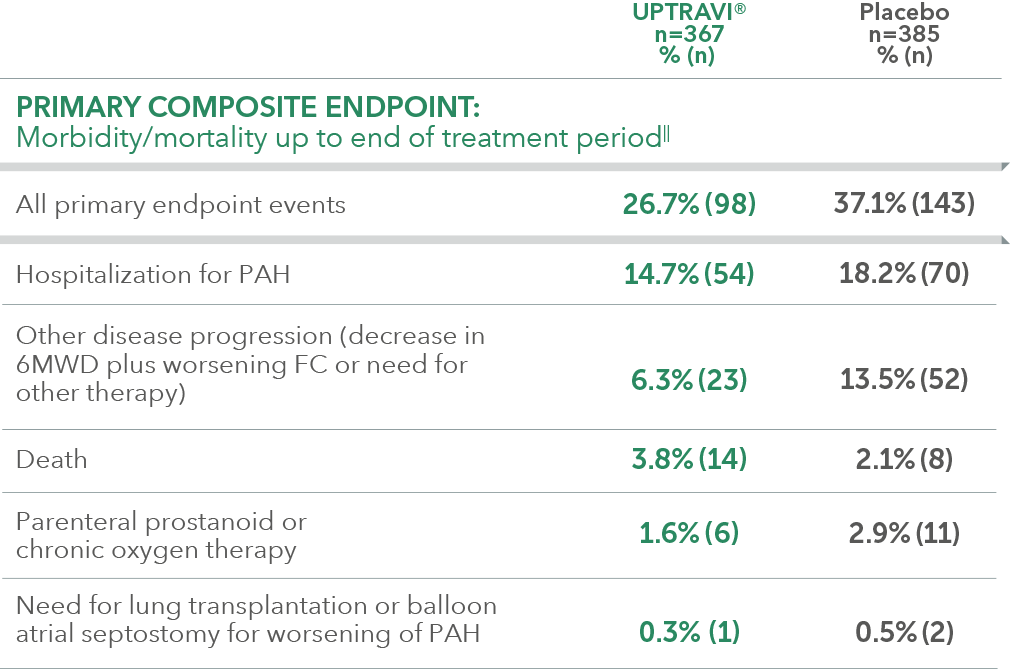
Summary of primary endpoint events in patients treated after 6 months of PAH diagnosis
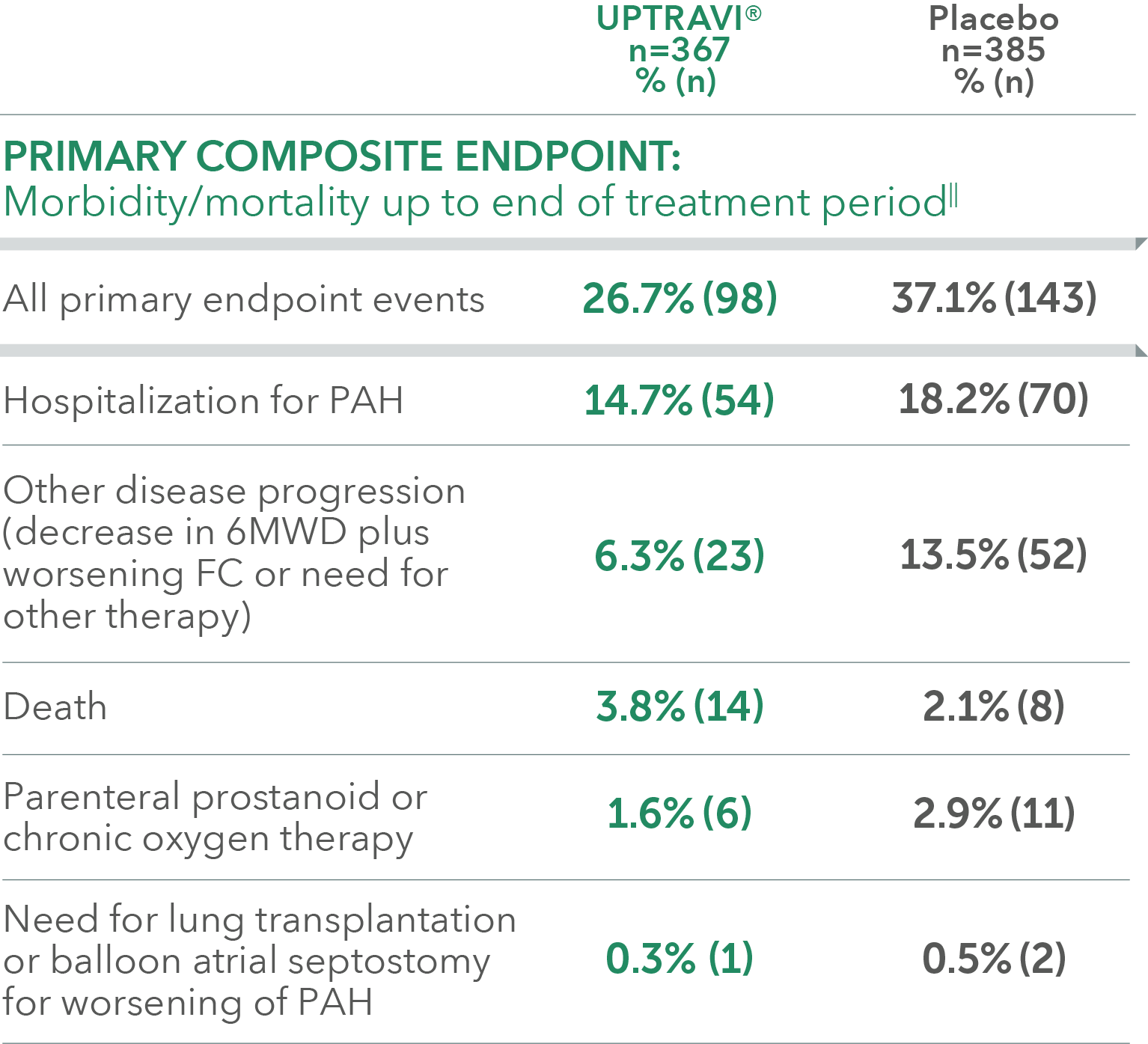
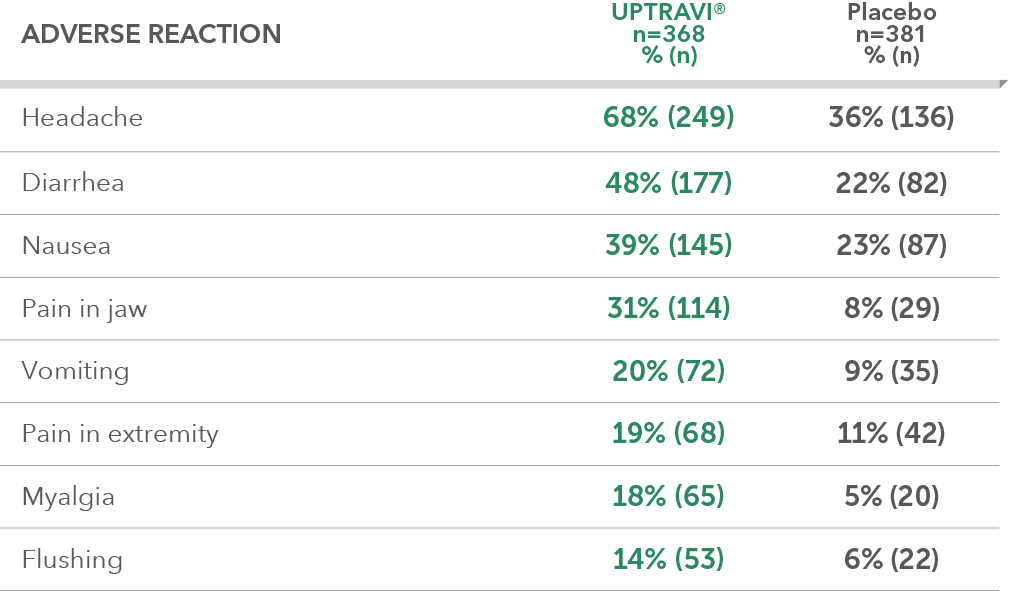
Adverse reactions in >6 months from diagnosis subgroup occurring more frequently with UPTRAVI® compared with placebo by ≥3%
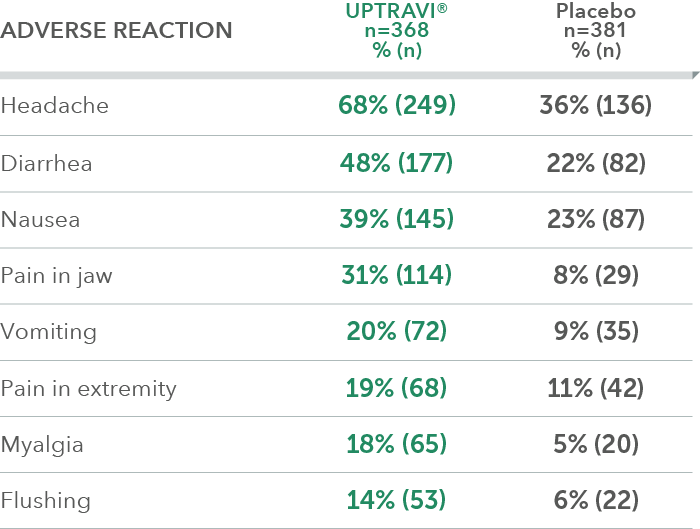
The analysis described here is post hoc and exploratory. The subgroup was not prespecified for evaluation of the primary endpoint. Please note this analysis did not compare patients treated within 6 months of PAH diagnosis with patients treated after 6 months of PAH diagnosis. Sample size should be considered and results should be interpreted with caution.