Adverse Reactions Ocurring More Frequently With UPTRAVI® Compared With Placebo By ≥3% in the GRIPHON Trial1
| ADVERSE REACTION | UPTRAVI® n=575 | Placebo n=577 |
|---|---|---|
| Headache | 65% | 32% |
| Diarrhea | 42% | 18% |
| Jaw pain | 26% | 6% |
| Nausea | 33% | 18% |
| Myalgia | 16% | 6% |
| Vomiting | 18% | 9% |
| Pain in extremity | 17% | 8% |
| Flushing | 12% | 5% |
| Arthralgia | 11% | 8% |
| Anemia | 8% | 5% |
| Decreased appetite | 6% | 3% |
| Rash | 11% | 8% |
Pivotal trial overall population: Adverse reactions were less frequent during the maintenance phase
•
Hyperthyroidism was observed in 1% (n=8) of patients on UPTRAVI® and in none of the patients on placebo
•
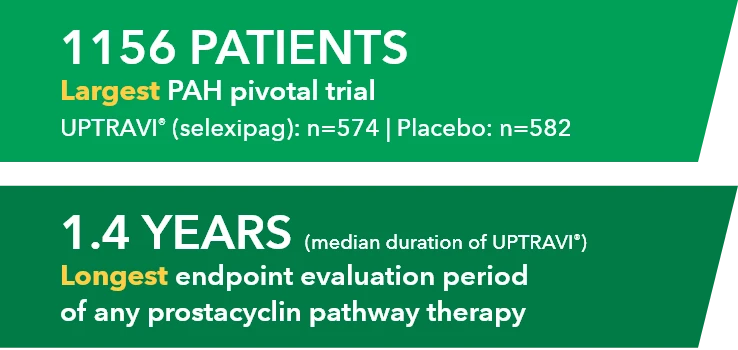
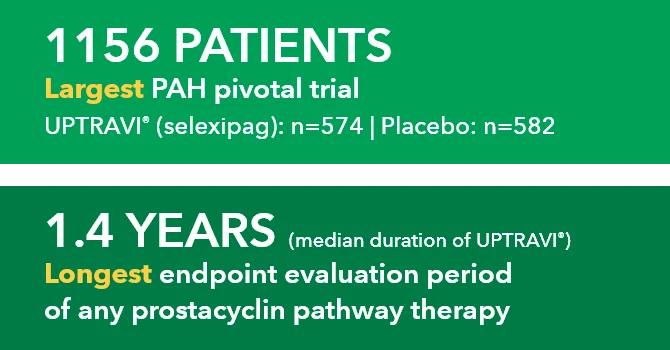
Median duration of exposure to UPTRAVI® was 1.4 years
Help Your Patients Be Prepared for Potential Adverse Reactions and How to Manage Them
Adverse Reactions Management Tool: Learn about adverse reactions common to prostacyclin-class therapies.
UPTRAVI® Dose Adjustment Phase Guide for patients: Use this guide for patients who are starting UPTRAVI® to help set goals and expectations for treatment. After discussion, give patients the guide to help them track and share how they’re feeling.