These data are from long-term follow-up and an open-label extension study. These uncontrolled observations do not allow comparison with a control group not given UPTRAVI® and cannot be used to determine the long-term effect of UPTRAVI® on mortality.
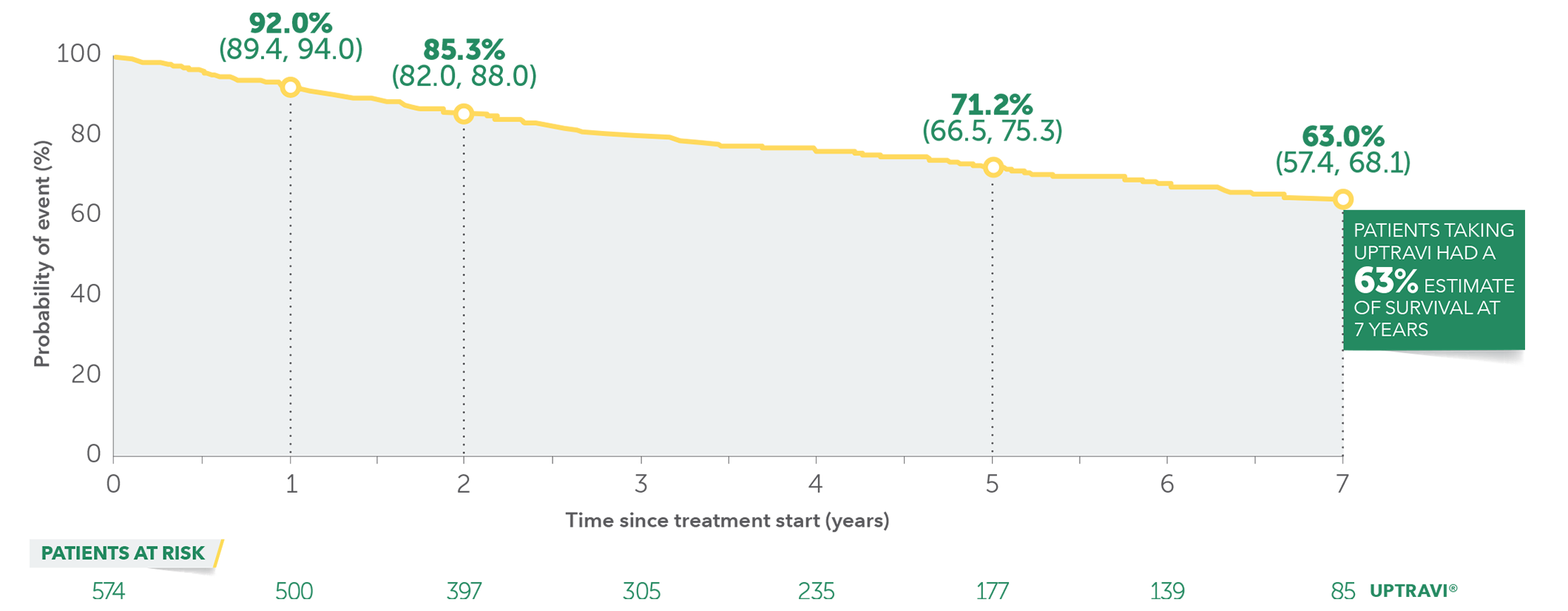
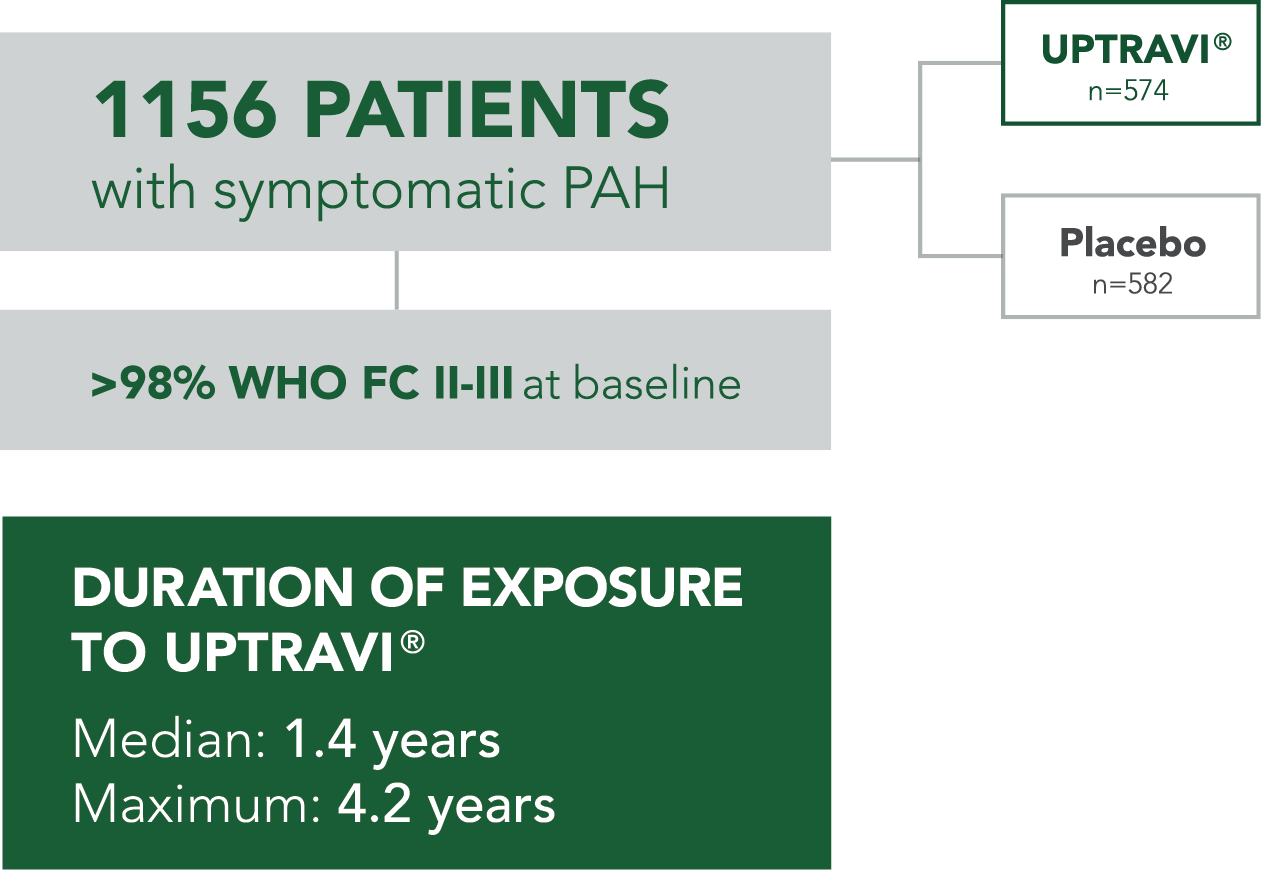
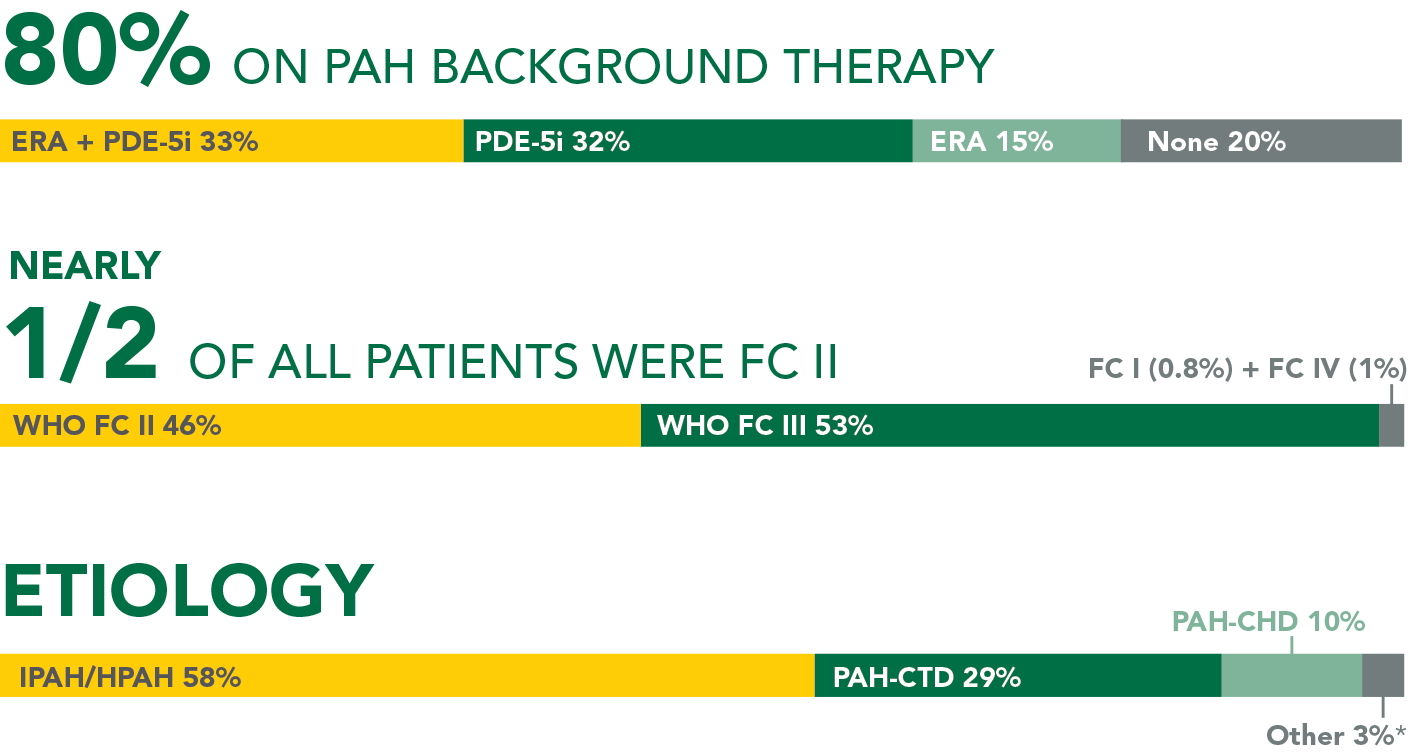
In long-term follow-up of patients who were treated with UPTRAVI® in the pivotal study and the open-label extension (N=574), Kaplan-Meier estimates of survival of these patients across the GRIPHON study and the long-term extension study at 1, 2, 5, and 7 years were 92%, 85%, 71%, and 63%, respectively. The median exposure to UPTRAVI® was 3 years.
Overall survival Kaplan-Meier curve for patients treated with UPTRAVI® in GRIPHON and entered into the open-label extension study1,3*
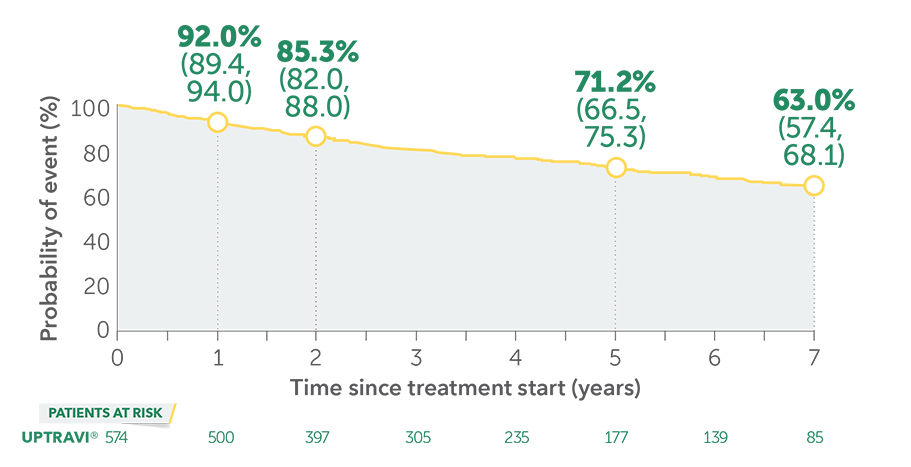
Data presented are Kaplan-Meier survival rate estimates (95% CI).