GRIPHON 10-YEAR OPEN-LABEL EXTENSION
GRIPHON Trial Overview
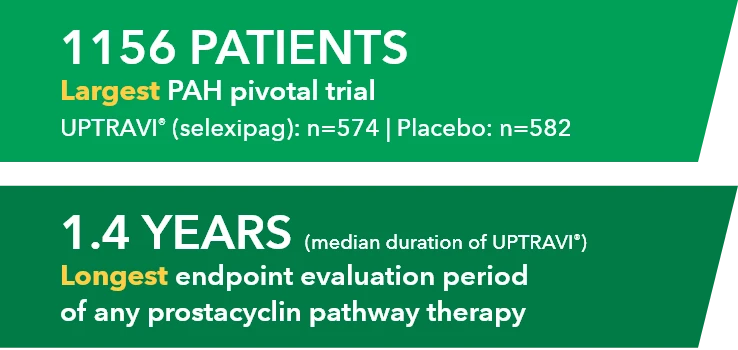
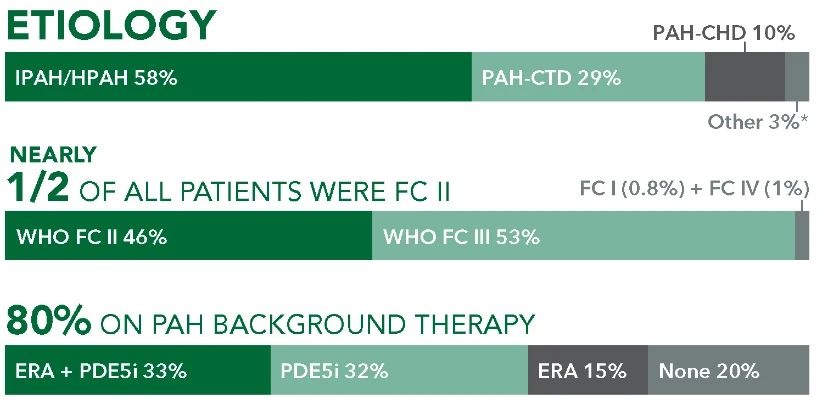
The safety and efficacy of UPTRAVI® (selexipag) were demonstrated in a multicenter, double-blind, placebo-controlled, parallel-group, event-driven study in patients with symptomatic PAH (>98% WHO FC II or III). The primary endpoint was the time to first disease progression event.1,2*
- Treatment with UPTRAVI® resulted in a 40% risk reduction† (99% CI: 22% to 54%; P<0.0001; HR 0.60) in disease progression compared with placebo (27% [155/574] vs 41.6% [242/582], respectively)1,2
- Adverse reactions occurring more frequently (≥5%) on UPTRAVI® compared with placebo are headache, diarrhea, jaw pain, nausea, myalgia, vomiting, pain in extremity, and flushing1
GRIPHON 10-Year OLE Overview
Limitations
These data are from a long-term follow-up and an open-label extension study. All analyses are descriptive only. Baseline characteristics were not balanced. These uncontrolled observations do not allow comparison with a control group not given UPTRAVI® and cannot be used to determine the long-term effect of UPTRAVI® on mortality.
Study Objectives
Assessments included patient characteristics, Kaplan-Meier survival estimates (assessed in the overall population, by individual maintenance dose, and by background therapy and time from diagnosis), and safety and tolerability.3
10-YEAR OLE: Overall Survival




Kaplan-Meier survival estimates (95% confidence intervals) are shown.


10-Year OLE: Survival by Time From Diagnosis




Kaplan-Meier survival estimates (95% confidence intervals) are shown. Does not include 112 patients in the overall population who did not have a PAH-specific background therapy at baseline.


10-Year OLE: Survival by Dose
A low personal dose of UPTRAVI® (200 mcg to 400 mcg BID) is equally effective as higher personal doses (up to 1600 mcg BID) in a 10-year open-label extension study3




Kaplan-Meier survival estimates (95% confidence intervals) are shown. Does not include 8 patients in the overall population who were on an individualized maintenance dose of UPTRAVI® <200 mcg BID and 1 patient whose individualized maintenance dose of UPTRAVI® (700/900 mcg BID) did not meet the criteria for “medium” dose.


UPTRAVI® demonstrated a consistent safety profile for over 10 years3
10 years of data: The longest follow-up in a clinical study of any PAH therapy3


| ADVERSE EVENT3 | UPTRAVI® N=574, n (%) |
|---|---|
| Headache | 390 (68) |
| Diarrhea | 265 (46) |
| Nausea | 209 (36) |
| Pulmonary arterial hypertension worsening | 203 (35) |
| Pain in jaw | 156 (27) |
| Death§ | 126 (22) |


Let real-world evidence inform your decisions
VIEW SPHERE REGISTRY