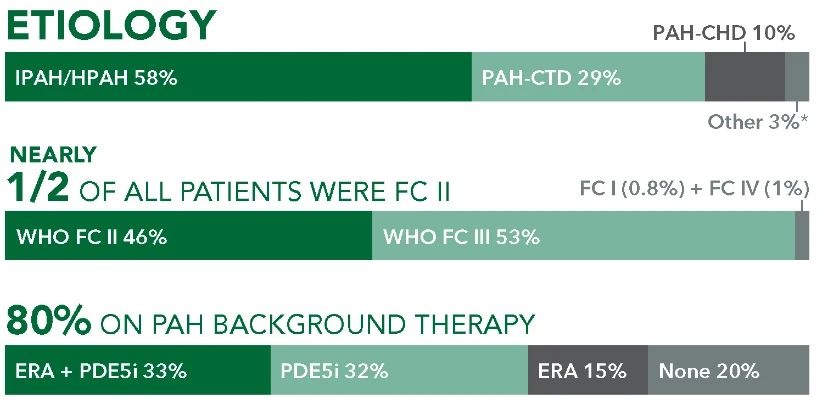
Studied in a Broad Range of Patients With PAH1
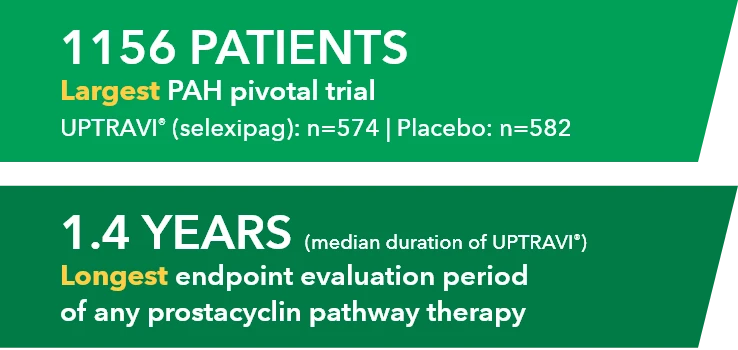
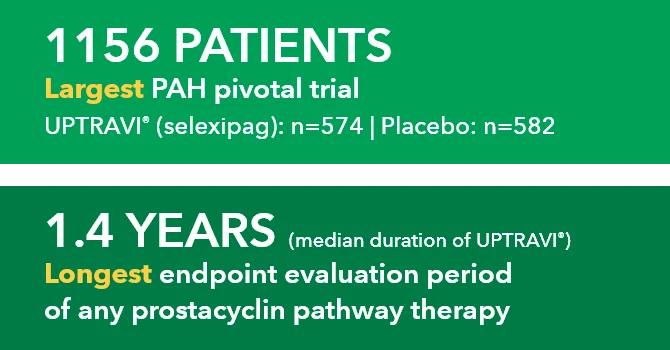
SPHERE (SelexiPag: tHe usErs dRug rEgistry) is the largest real-world registry of a prostacyclin pathway agent to date in the US1-4*
Study Design1
Patients aged ≥18 years were enrolled from November 2016 to March 2020 and followed for up to 18 months. UPTRAVI® was initiated by the treating physician per routine clinical practice.† There were no study-mandated visits or procedures. Data were collected at enrollment and every 3 months thereafter via electronic case report. AEs were collected from enrollment to the last UPTRAVI® dose. During the titration phase, AEs associated with the mode of action of UPTRAVI® were collected only if they were defined as serious, led to discontinuation of UPTRAVI®, or reflected an unusual pattern of severity according to investigator judgment.1
Study Limitations and Considerations1
- The main limitations of this study are related to its observational nature, including: the potential bias introduced by including previously initiated patients for whom no data were collected between treatment initiation and study enrollment, incomplete records reflective of clinical practice can impact interpretation of findings, and the data analyses reported are descriptive only. No clinical conclusions can be drawn due to these limitations of this study
- As a drug registry (rather than a disease registry), SPHERE recruited only patients receiving UPTRAVI®, who typically take UPTRAVI® as part of combination therapy. This introduced bias in favor of combination therapy recipients
- The following may contain data, conclusions, and recommendations that do not conform to the US FDA-approved labeling for the product discussed herein. UPTRAVI® should be used only as specified in the Prescribing Information for UPTRAVI®. Due to the real-world nature of the study, patients receiving UPTRAVI® who had PH but not PAH also participated; however, for the present analysis, we have focused on patients with PAH. Please see the full Prescribing Information for UPTRAVI® for adequate directions for use for the approved indication


759 Patients
Newly
initiated‡: 387
Previously initiated§: 372
Median age at UPTRAVI® initiation:
61 years
Female: 76.5%
2.7 years
Median time from PAH diagnosis to initiation of UPTRAVI®
57.3%
Intermediate or high risk at baseline per REVEAL 2.0
80.5%
WHO FC II or III


Changes in Risk Status and Functional Class












Overall Survival Rate in Patients With PAH






These uncontrolled observations do not allow comparison with a control group not given UPTRAVI® and cannot be used to determine the effect of UPTRAVI® on mortality.
Safety in the SPHERE Registry


| Adverse Event | All patients N=759 |
|---|---|
| Any AE | 543 (71.5%) |
| Serious AE | 278 (36.6%) |
| AE leading to death | 54 (7.1%) |
| AE leading to hospitalization | 262 (34.5%) |
| AE leading to discontinuation | 188 (24.8%) |
| Related to UPTRAVI® | 53 (7.0%) |
| Headache | 23 (3.0%) |
| Diarrhea | 11 (1.4%) |
| Myalgia | 12 (1.6%) |
| Nausea | 12 (1.6%) |
| Arthralgia | 6 (0.8%) |
| Pain in jaw | 4 (0.5%) |
| Adverse Event | All patients N=759 |
|---|---|
| Related to PAH progression | 107 (14.1%) |
| Pulmonary hypertension | 18 (2.4%) |
| Dyspnea | 15 (2.0%) |
| Right ventricular failure | 15 (2.0%) |
| Pulmonary arterial hypertension | 13 (1.7%) |
| Acute respiratory failure | 10 (1.3%) |
| Respiratory failure | 6 (0.8%) |


Start your PAH patients on UPTRAVI®
EXPLORE DOSING