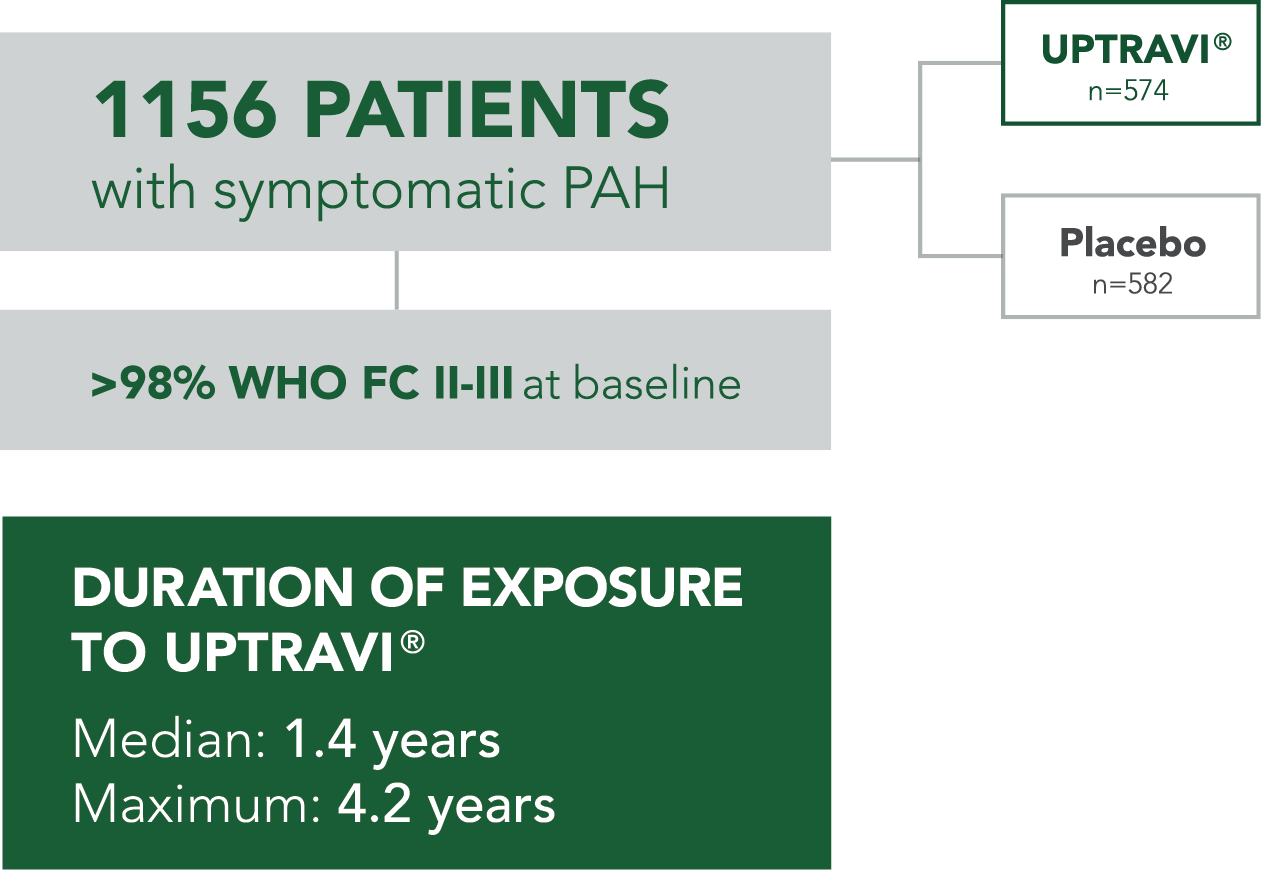
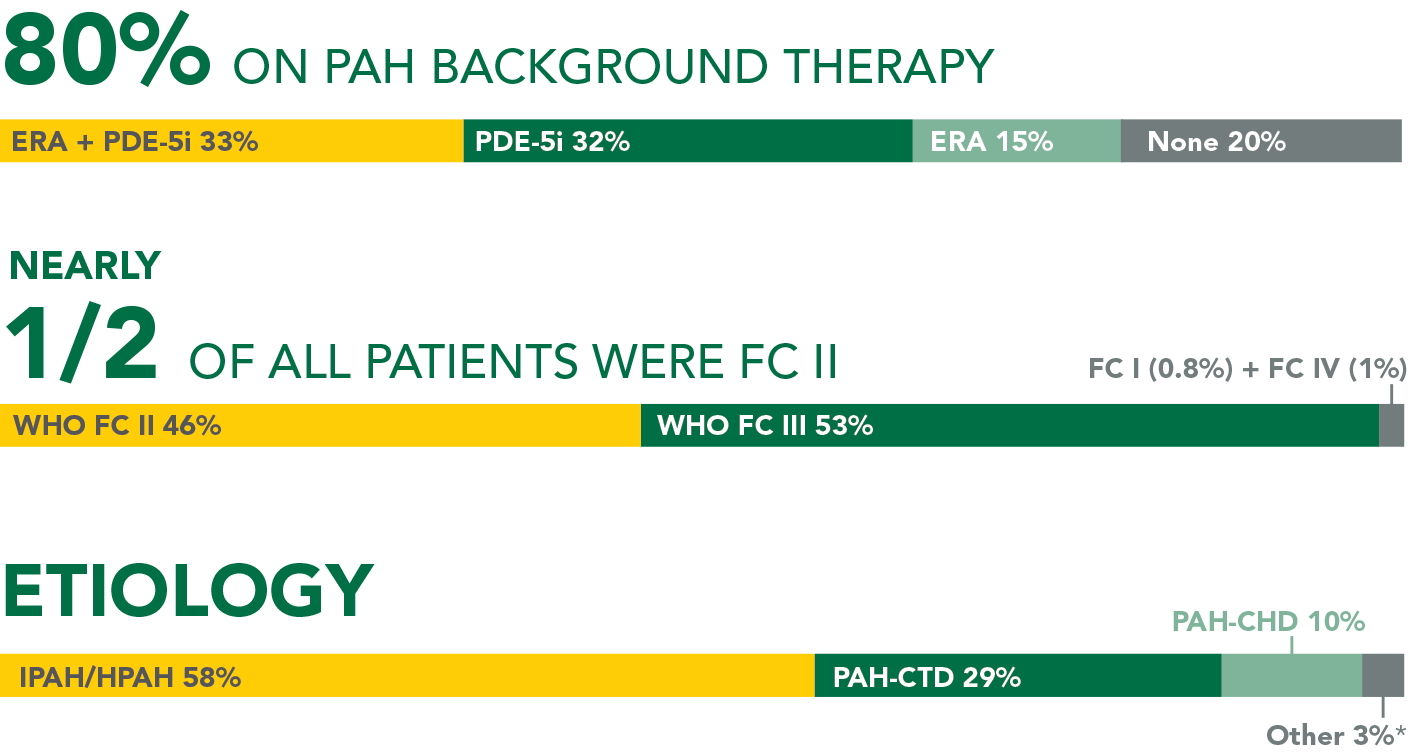
GRIPHON: THE LARGEST OUTCOMES TRIAL CONDUCTED IN PAH (N=1156)1-3
The safety and efficacy of UPTRAVI® was demonstrated in a multicenter, double-blind, placebo-controlled, parallel-group, event-driven study in patients with symptomatic PAH (>98% WHO FC II or III). The primary endpoint was the time to first disease progression event.* Treatment with UPTRAVI® resulted in a 40% risk reduction† (99% CI: 22% to 54%; P<0.0001; HR 0.60) in disease progression compared with placebo (27% [155/574] vs 41.6% [242/582], respectively). Adverse reactions occurring more frequently (≥5%) on UPTRAVI® compared with placebo are headache, diarrhea, jaw pain, nausea, myalgia, vomiting, pain in extremity, and flushing.
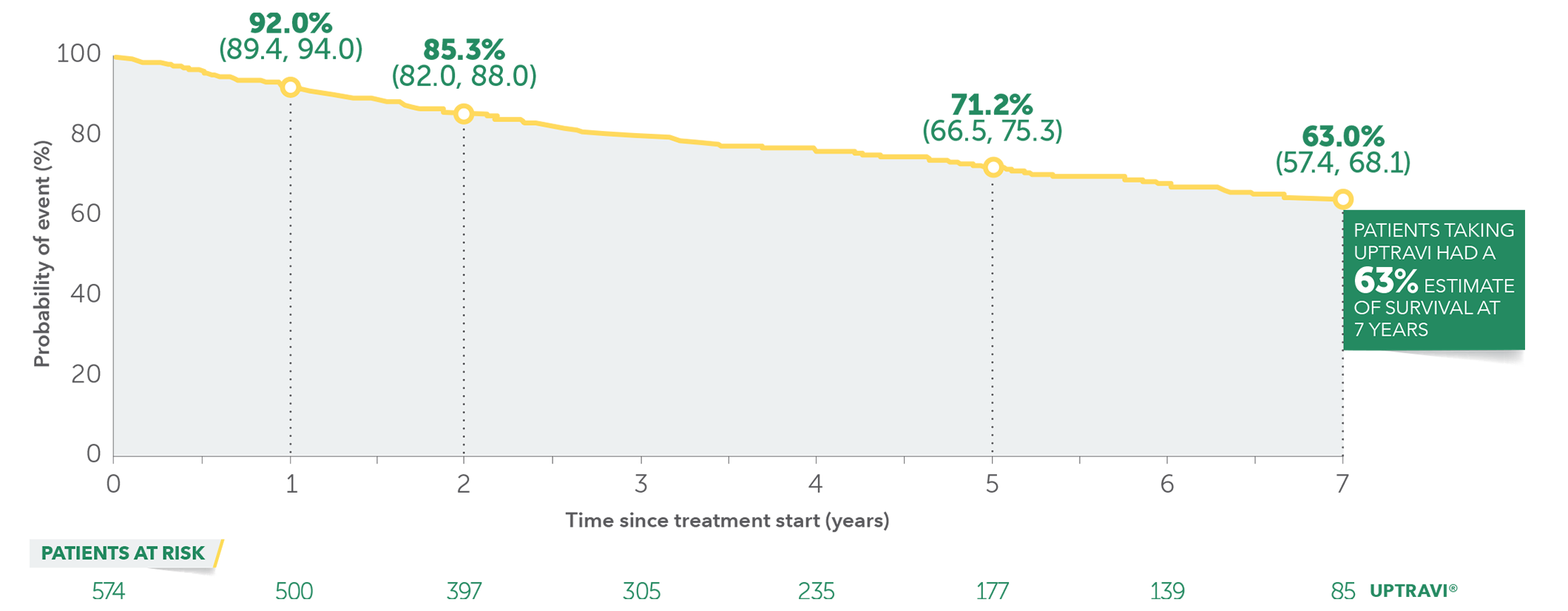
UPTRAVI®—The Only Oral Prostacyclin Pathway Therapy With 7-year Survival Estimates1,4
These data are from long-term follow-up and an open-label extension study. These uncontrolled observations do not allow comparison with a control group not given UPTRAVI® and cannot be used to determine the long-term effect of UPTRAVI® on mortality.
In long-term follow-up of patients who were treated with UPTRAVI® in the pivotal study and the open-label extension (N=574), Kaplan-Meier estimates of survival of these patients across the GRIPHON study and the long-term extension study at 1, 2, 5, and 7 years were 92%, 85%, 71%, and 63%, respectively. The median exposure to UPTRAVI® was 3 years.
Overall survival Kaplan-Meier curve for patients treated with UPTRAVI® in GRIPHON and entered into the open-label extension study1,5‡
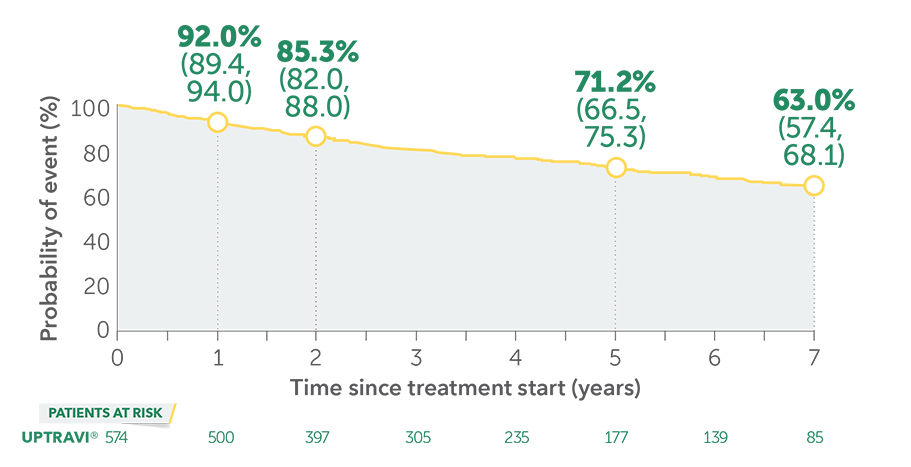
Data presented are Kaplan-Meier survival rate estimates (95% CI).